International Relations
FATF’s Grey List
The Financial Action Task Force (FATF), after its 5-day plenary (concluded on 19th October 2019 in Paris), decided to keep Pakistan on the Grey List.
- However, it has warned that Pakistan will be put on the Black List if it does not control terror funding by February 2020.
- It has been reported that the immediate blacklisting of Pakistan did not garner the consensus of all FATF members.
- India led a diplomatic offensive against Pakistan for its blacklisting. France, the US and the European Union countries supported India.
- China, Turkey and Malaysia resisted India’s efforts.
Financial Action Task Force
- The Financial Action Task Force (FATF) is an inter-governmental body established in 1989 during the G7 Summit in Paris.
- The objectives of the FATF are to set standards and promote effective implementation of legal, regulatory and operational measures for combating money laundering, terrorist financing and other related threats to the integrity of the international financial system.
- Its Secretariat is located at the Organisation for Economic Cooperation and Development (OECD) headquarters in Paris.
- Member Countries: As of 2019, it consists of thirty-seven member jurisdictions. India is one of the members.
- FATF has two lists:
- Grey List: Countries that are considered safe haven for supporting terror funding and money laundering are put in the FATF grey list. This inclusion serves as a warning to the country that it may enter the blacklist.
- Black List: Countries known as Non-Cooperative Countries or Territories (NCCTs) are put in the blacklist. These countries support terror funding and money laundering activities. The FATF revises the blacklist regularly, adding or deleting entries.
- The FATF Plenary is the decision making body of the FATF. It meets three times per year.
FATF & Pakistan
- Pakistan was placed on the grey list by the FATF in June 2018 and was given a plan of action to complete by October 2019, or face the risk of being placed on the black list with Iran and North Korea.
- Pakistan was previously placed on the FATF's grey list in February 2012, and had been removed from the grey list in February 2015 after it passed a National Action Plan (NAP) to deal with terrorism after the Peshawar School massacre in December 2014.
- It was placed under severe restrictions in the years 2008-2012.
FATF’s latest Review of Pakistan
- Pakistan addressed only 5 of the 27 tasks given to it to control funding to terror groups like the Lashkar-e-Taiba and Jaish-e-Mohammad — both responsible for a series of attacks in India.
- The FATF noted the insufficiency of Pakistan’s implementation as “serious concerns”.
- The latest decision to keep the country in ‘Grey List’ means that Pakistan has been given time until February 2020 to fulfil its commitments or risks being blacklisted.
- The main purpose behind the decision is to not punish rather than incentivise, to make the required changes and make them faster.
- Impact on Pakistan:
- By remaining on the “Grey List”, it would be difficult for Pakistan to get financial aid from the International Monetary Fund (IMF), World Bank and European Union, making its financial condition more precarious.
- However, there are no immediate implications for the recent $6 billion loan negotiated with the IMF that is to be disbursed over the next three years.
- The country is facing a number of economic challenges with its economy expected to grow at 3.3 % in 2019 and 2.6% in 2020, according to IMF.
- Inflation is set to touch 7.3% in 2019, up from 3.9% in 2018, and rise to 13% in 2020.
- Fiscal deficit is projected at 7.1% of GDP in 2020, the highest in the last seven years.
- By remaining on the “Grey List”, it would be difficult for Pakistan to get financial aid from the International Monetary Fund (IMF), World Bank and European Union, making its financial condition more precarious.
Science & Technology
World Intellectual Property Indicators- 2019
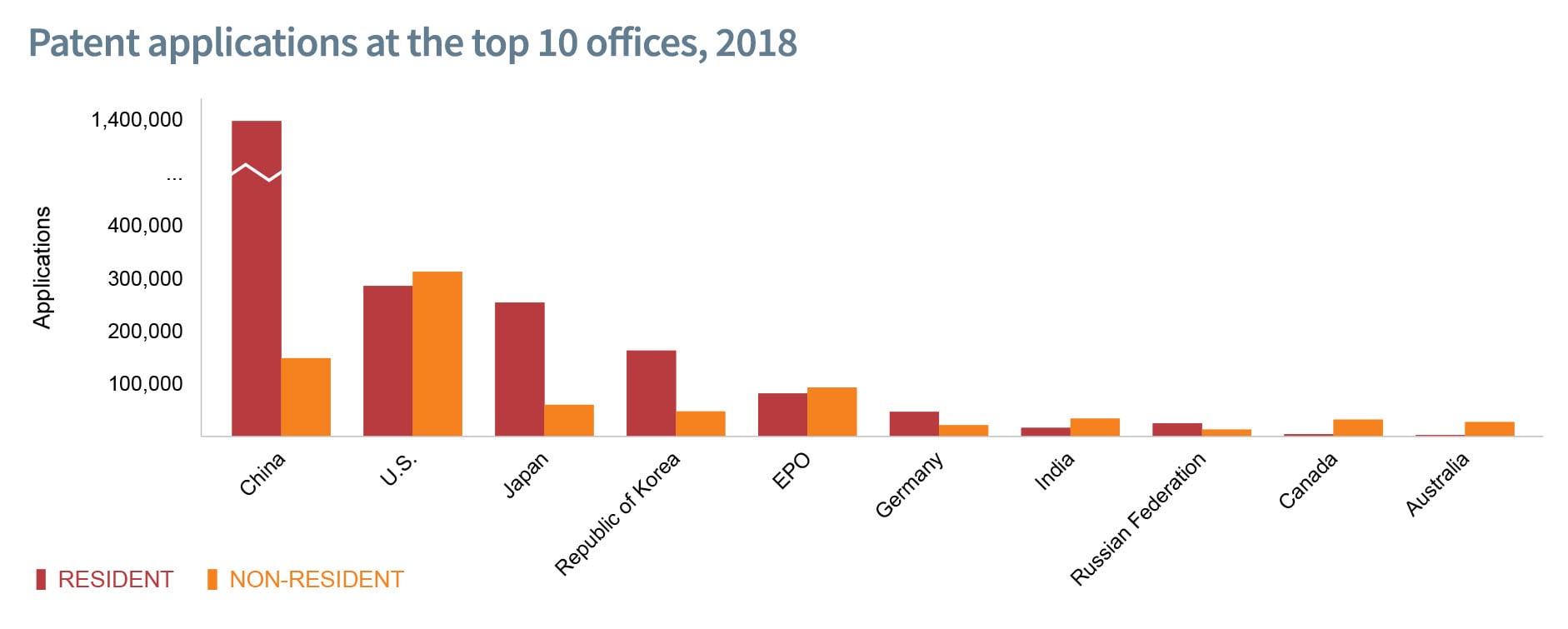
According to the United Nations, World Intellectual Property Indicators Report-2019, global Intellectual Property (IP) filing activity continued to grow at a rapid pace.
- In 2018, patent filings around the world exceeded by around 3.3 million, representing a 5.2% growth over 2017 figures.
- Asia accounted for two-thirds of these applications, being the global hub for IP applications.
- China has been at the leading position of global growth in worldwide IP filings in 2018 whereas the United States of America marked its first decline since 2009.
- In India, there was a large increase in the number of filings with respect to
- Trademark filing activity (+20.9%),
- Patent filings (+7.5%), and
- Industrial design filing activity (+13.6%), marking almost double-digit growth in 2018. For India, this was the third successive year of double-digit growth.
- Amongst the middle-income countries in the period from 2015 to 2017, applicants residing in India (16.8% of total published applications) mostly filed the patents related to the pharmaceuticals sector.
- The share of withdrawn or abandoned applications was also highest in India (66.2%).
- However, India reduced the number of pending applications by 25% in 2018 compared over the last year (2017).
- Trade Mark: A trademark is a word, phrase, symbol, and/or design that identifies and distinguishes the source of the goods of one party from those of others. The term "trademark" is often used to refer to both trademarks and service marks. Unlike patents and copyrights, trademarks do not expire after a set term of years. Instead, a trademark can last forever, so long as the owner continues to use the mark in commerce to indicate the source of goods and services.
- Patents: A patent is a limited duration property right relating to an invention, granted by the Trademark Office in exchange for public disclosure of the invention. Patentable materials include machines, manufactured articles, industrial processes, and chemical compositions. The duration of patent protection is 20 years in the case of India.
- Copyright: A copyright protects works of authorship that have been tangibly expressed in a physical form. Example - songs, books, movies, and works of art.
- Industrial Design: in a legal sense, an industrial design constitutes the ornamental or aesthetic aspect of an article. It may consist of three-dimensional features, such as the shape of an article, or two-dimensional features, such as patterns, lines or colours.
Science & Technology
Aflatoxin-M1 in Processed Milk
Recently the Food Safety and Standards Authority of India (FSSAI) has tested different types of milk (raw and processed of various types) samples under the National Milk Safety and Quality Survey 2018.
- This is the first-of-its-kind comprehensive survey FSSAI has conducted through a third-party agency.
- The survey was conducted from May 2018 to October 2018 covering all states and UTs.
- It covered both organised (retailers and processors) as well as non-organised (local dairy farms, milk vendors and milk mandis) sectors.
Findings of the Study
- About 93% of the milk samples tested were found to be safe for consumption. Remaining 7% were found to have the presence of contaminants such as Aflatoxin-M1, pesticides and antibiotics.
- Contrary to common perception, the study shows that contamination was a more serious problem than adulteration.
- Chemical contaminants in milk comprise chemical hazards that may be introduced during milk production, dairy processing or packaging.
- Veterinary drugs, heavy metals, radionuclides, mycotoxins and pesticides are chemical contaminants that can enter to animal feed and thereby in milk.
- The most common contaminant in milk is antimicrobial drugs.
- An adulteration is an act of intentionally degrading the quality of food either by the admixture or substitution of inferior substances or by the removal of some valuable ingredient.
- Chemical contaminants in milk comprise chemical hazards that may be introduced during milk production, dairy processing or packaging.
- The problem of Aflatoxin-M1 was found to be more dominant in processed milk than raw milk.
- Aflatoxin-M1 comes in the milk through feed and fodder which are currently not regulated in the country.
- Tamil Nadu, Delhi and Kerala were the top three states where Aflatoxin residue was found the maximum.
- In terms of quality, the survey found that 37.7% of the total sample of processed milk did not comply with quality parameters because the presence of contaminants such as fats, SNF (solids not fat), Maltodextrin and sugar was above the permissible limits.
- Adulteration was found in only 12 of the total samples surveyed with a maximum reported in Telangana, followed by Madhya Pradesh and Kerala.
Aflatoxin-M1
- Aflatoxins are toxins produced by certain fungi which are generally found in agricultural crops like maize, peanuts, cotton seed and others. They are carcinogenic in nature.
- According to a World Health Organization (WHO) study, consumption of food containing aflatoxin concentrations of one milligram per kilogram or higher has been suspected to cause aflatoxicosis, the outcome of which consists of acute liver failure, jaundice, lethargy and nausea, eventually leading to death
- The exposure to AFM1 from milk causes stunting among children.
Stunting
- It is the impaired growth and development that children experience from poor nutrition, repeated infection, and inadequate psychosocial stimulation.
- Children are defined as stunted if their height-for-age is more than two standard deviations below the WHO Child Growth Standards median.
- In India, 38% of children younger than five years of age are stunted, a manifestation of chronic undernutrition. Stunting and other forms of under-nutrition are responsible for nearly half of all child deaths globally.
Social Justice
Draft Notification for Medical Implants
Recently, a draft notification issued by the Ministry of Health and Family Welfare has proposed to bring "all devices" used for medical application, under the purview of Drugs and Cosmetics Act, 1940. This will make “all devices” to be termed as “drugs” .
- All devices will include medical instruments, apparatus, appliance, implant, material or other article, whether used alone or in combination, including software or an accessory, to be used especially for human beings or animals.
- Manufacturers and importers of most of these devices will have at least 1.5 years to voluntarily register with the Central Drugs Standard Control Organisation.
The Rationale Behind the Draft Notification
- Defective implants can cause crippling pain and even death. For example, Johnson and Johnson’s faulty hip implants.
Johnson and Johnson’s faulty hip implants case
- In 2018, Johnson and Johnson’s hip implant called Pinnacle was found to be leaking the cobalt-chromium ions into the body, leading to serious health complications, including metal poisoning of the blood, debilitating pain, and damage to the body organs.
- Further, Johnson and Johnson have paid compensations to US patients who had received the defective implants. However, In India, the company has challenged government orders to compensate 4,700 patients who had undergone hip replacement surgeries.
- Therefore, the Johnson and Johnson continue to exploit the regulatory deficit in India.
Impact
- If implemented, the country’s drug regulator will enforce standards to ensure the safety and effectiveness of these products while its pricing regulator will monitor the prices.
- Central Drugs Standard Control Organisation (CDSCO) is drug regulator in India.
- It applies the provisions of the Drugs and Cosmetics Act, as well as the Medical Devices Rules 2017 on all medical devices.
- It can also punish for violations as per the Act.
- While, the National Pharmaceutical Pricing Authority (NPPA), monitor the prices of drugs and ensure that they don’t raise it more than 10% every year.
- Central Drugs Standard Control Organisation (CDSCO) is drug regulator in India.
Way Forward
- The application of these medical regulations is marred by inordinate delays.
- For example, the Food and Drug Administration of Maharashtra had directed to stop the import of Johnson and Johnson’s hip implants, few months after Johnson and Johnson withdrew the product from the global market.
- However, it took another year for the Central Drugs Standard Control Organisation to ban the import.
- Therefore, merely expanding the scope of regulation to all devices is not enough in a moment of growing number of safety disasters involving devices.
- So, there is a pressing need for framing of a new medical devices act.
Central Drugs Standard Control Organisation (CDSCO)
- The CDSCO is the Central Drug Authority for discharging functions assigned to the Central Government under the Drugs and Cosmetics Act.
- Major Functions:
- Regulatory control over the import of drugs, approval of new drugs and clinical trials.
- Approval of certain licences as Central Licence Approving Authority
National Pharmaceuticals Pricing Authority
- NPPA is an organization under Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers which was set up in 1997 to revise the prices of controlled bulk drugs and formulations and to enforce prices and availability of medicines in the country, under the Drugs (Prices Control) Order (DPCO), 1995.
- The prices are now fixed/revised under Drugs (Prices Control) Order (DPCO), 2013.
- It also monitors the prices of decontrolled drugs in order to keep them at reasonable levels.
Governance
Nuclear Energy Conclave
The 11th Nuclear Energy Conclave, organized by the India Energy Forum, was held in New Delhi on 18th October 2019.
- The theme of the Conclave was: “Economics of Nuclear Power- Innovation towards Safer & Cost Effective Technologies”.
Key Highlights
- The Minister of State for Atomic Energy highlighted some steps taken by the Government with respect to nuclear energy:
- Earlier the Atomic power plants were restricted in Southern India, now the Government is setting up the nuclear plants in other parts of the country.
- A nuclear plant is coming up in the Gorakhpur of Haryana.
- A “Hall of Nuclear Energy” was opened in in Delhi to educate the students and the general public about the applications of nuclear energy.
- Use of nuclear energy in diverse fields such as medicine, especially cancer care.
- Other steps highlighted include early movement on Fast Breeder Reactors (FBR) deployment and early deployment of indigenous Light Water Reactors (LWRs).
- Light-water reactors (LWRs) are power reactors that are cooled and moderated with ordinary water. There are two basic types: the Pressurized-Water Reactor (PWR) and the Boiling-Water Reactor (BWR).
- PWR is a power reactor in which the heat is dissipated from the core using highly pressurized water (about 160 bar) to achieve a high temperature and avoid boiling within the core. BWR is a nuclear reactor with water as a coolant and as a moderator, boiling in the core. The resulting steam is generally used directly to drive a turbine.
- It was underlined that owing to the waiver of the Nuclear Suppliers Group (NSG) to India in 2008, the nuclear programme now has much less constraints.
- Access to the imported uranium can accelerate the nuclear program size as well as large scale thorium deployment.
India Energy Forum
- Established in October 2001, the Forum has acquired a unique status as a spokesman of total energy sector.
- Major public and private sector organizations in Power, Oil and Gas, Coal and Renewable Energy are its members. These include NTPC, NHPC, Power Grid Corporation, Power Finance Corporation, ONGC, etc.
- It’s Corporate Office is located in New Delhi.
India’s Nuclear Energy Programme
- The nuclear energy programme in India was launched around the time of independence under the leadership of Homi J. Bhabha.
- The main objectives of the Indian Nuclear Energy programme are to provide safe and reliable electric power for the country’s social and economic progress and to be self reliant in all aspects of nuclear technology.
- Exploration of atomic minerals in India, undertaken since the early fifties, has indicated that India has limited reserves of uranium (natural uranium consists of mostly 238U, with 0.7 % 235U), but fairly abundant reserves of thorium (232Th).
- Accordingly, India has adopted a three stage strategy of nuclear power generation:
- Stage 1 Pressurised Heavy Water Reactor (PHWR): The PHWR is a pressure tube type reactor using heavy water (D2O) moderator, heavy water coolant and natural uranium dioxide fuel. Considering the growing energy demands and the necessity to increase the energy potential, a second line of light water reactors have been added to the current indigenous programme of Pressurised Heavy Water Reactors.
- Stage 2 Fast Breeder Reactor (FBR): India’s second stage of nuclear power generation uses the Pu-239 obtained from the first stage reactor operation, as the main fissile element of fuel core in fast breeder reactors (FBR). A blanket of U-238 surrounding the fuel core undergoes nuclear transmutation to produce fresh Pu-239 as more and more Pu-239 is consumed during the operation. The Fast Breeder Programme is in the technology demonstration stage. Sodium, because of its good heat transfer and nuclear properties, is used as the coolant to remove the heat generated in the reactor.
- Stage 3 Thorium based Reactor: Thorium utilization is the long term objective of the Indian Nuclear Power Programme. The third phase of India’s Nuclear Power Generation programme is breeder reactors based on Thorium- Uranium-233 cycle.
Important Facts For Prelims
Danx-19
Recently, the Andaman and Nicobar Command (ANC) has conducted the second edition of Defence of Andaman & Nicobar Islands 2019 (DANX-19).
- Indian Army, Navy, Air Force, Coast Guard and special forces from newly formed Armed Forces Special Operations Division (AFSOD), participated in this exercise.
- The exercise was carried out for mobilisation and field manoeuvres to validate defensive plans of ANC headquarters and ensuring territorial integrity of the A&N Islands.