Governance
Paediatric Drug Regulation Framework in India
- 27 Oct 2025
- 19 min read
This editorial is based on “Respect the health rights of India’s children”, which was published in The Hindu on 25/10/2025. The article argues that India must urgently strengthen its regulatory, ethical, and research frameworks for paediatric medicines to safeguard children’s health rights and prevent tragedies like the deaths of 25 children due to contaminated cough syrup in Madhya Pradesh.
For Prelims: Central Drugs Standard Control Organisation (CDSCO), Pharmacovigilance Programme of India (PvPI), National Pharmaceutical Pricing Authority (NPPA), Drugs and Cosmetics Act, 1940, Pre-Conception and Pre-Natal Diagnostic Techniques (PCPNDT) Act, UN Convention on the Rights of the Child, Pradhan Mantri Bhartiya Janaushadhi Pariyojana (PMBJP), Strengthening of Pharmaceuticals Industry Scheme (SPI Scheme)
For Mains: The Constitutional and Policy Framework Governing Paediatric Medicines in India, The Critical Challenges in Regulating Paediatric Medicines in India, The Government Initiatives to Boost the Pharmaceutical Sector in India
In recent times, the tragic deaths of 25 children in Madhya Pradesh due to contaminated cough syrup have shaken the nation, exposing glaring gaps in India’s child healthcare and drug safety systems. With children forming nearly 40% of the population, ensuring their access to safe and effective medicines is a pressing concern. Weak regulatory oversight, lack of child-specific research, and insufficient pharmacovigilance highlight the urgent need for robust policies to protect India’s youngest and most vulnerable citizens.
What is the Constitutional and Policy Framework Governing Paediatric Medicines in India?
- Constitutional Provisions:
- Article 21: Guarantees the right to life, interpreted to include the right to health, making the state responsible for safe healthcare and medicines for children.
- Article 39(f) (Directive Principles of State Policy): Directs the State to secure that children are given opportunities to develop healthily and be protected from exploitation and neglect.
- Article 47: Directs the state to raise nutrition and living standards, linking child health to public health obligations.
- Policy and Legal Measures:
- Drugs and Cosmetics Act, 1940 & Rules, 1945: Core legal framework regulating drug manufacture, quality, and clinical trials, including ethical protocols for paediatric testing (Schedule Y).
- National Policy for Children, 2013: The policy identified Survival, Health, Nutrition, Education, Development, Protection, and Participation as the undeniable rights of every child.
- National Health Policy, 2017: The Policy aims to attain the highest possible level of health and well-being for all at all ages through preventive and promotive healthcare and universal access to quality health services.
- National Ethical Guidelines for Biomedical Research Involving Children (ICMR, 2017 update): Sets standards for research involving minors, including parental consent, minimal risk, and ethics oversight.
- Juvenile Justice Act,2015 : It is a legal framework in India designed to protect and provide care for children who are either in conflict with the law or are in need of care and protection due to abuse, neglect, or abandonment.
- UN Convention on the Rights of the Child (UNCRC, 1992): India’s ratification obligates the state to ensure the highest attainable health standards and safe medical treatment for children.
- Institutional and Regulatory Framework:
- Central Drugs Standard Control Organisation (CDSCO): Acts as India’s national drug regulator responsible for overseeing drug quality, safety, and approvals.
- Indian Pharmacopoeia Commission (IPC): It is an autonomous institution under the Ministry of Health and Family Welfare, Government of India.
- It is responsible for developing and updating the Indian Pharmacopoeia (IP) the official compendium of drug quality standards in India.
- Pharmacovigilance Programme of India (PvPI): Responsible for monitoring adverse drug reactions (ADRs) across India and identifying drug safety signals.
- General Drugs Control Inspectorate (GDCI): Supports CDSCO and state authorities in carrying out compliance checks, risk-based inspections, and sample testing across drug manufacturing units and retail outlets.
- Vital in the detection and reporting of substandard or spurious drugs flagged by CDSCO and in facilitating enforcement actions.
- National Pharmaceutical Pricing Authority (NPPA): NPPA regulates prices of essential medicines, enforces price caps, and monitors cost compliance across pharma companies to ensure affordability.
- Works with CDSCO to ensure essential paediatric formulations remain accessible and affordable, particularly after safety incidents that affect product availability.
What are the Critical Challenges in Regulating Paediatric Medicines in India?
- Inconsistent and Weak Regulatory Oversight: India’s regulatory fragmentation between the Central Drugs Standard Control Organisation (CDSCO) and various state drug controllers continues to cause enforcement gaps.
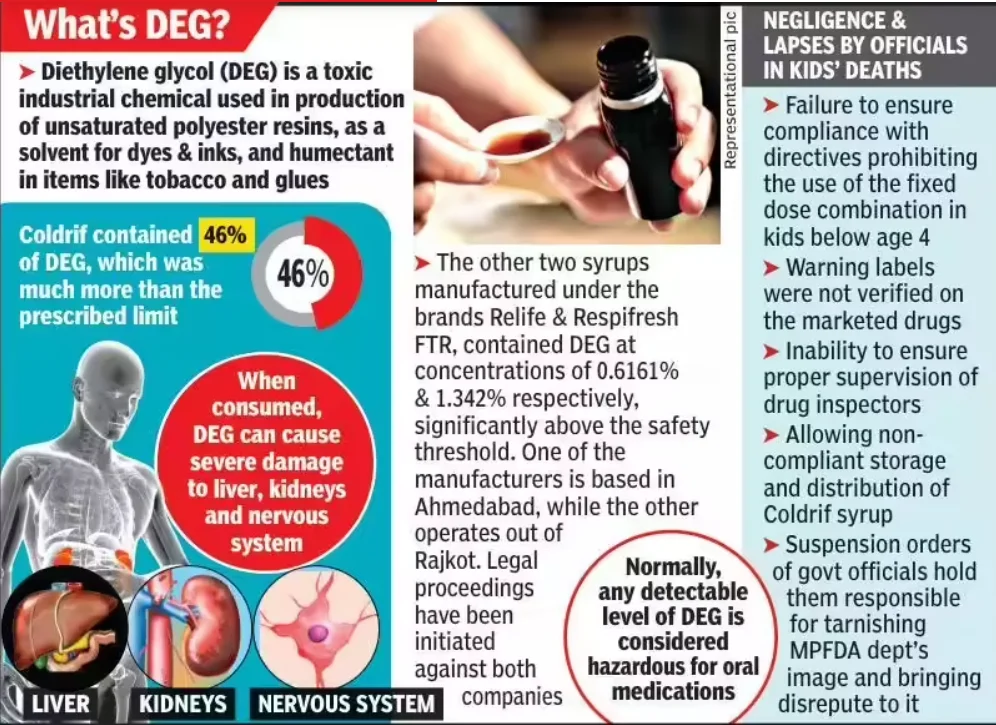
- During the 2025 Madhya Pradesh cough syrup crisis, regulators found 364 violations at Sresan Pharmaceuticals, the firm producing Coldrif, which contained diethylene glycol (DEG), a toxic substance found in industrial solvents.
- While the CDSCO tightened export testing rules, no similar mandate exists for domestic sales, leaving millions of Indian children vulnerable to unsafe medicines.
- A proposed Centralized Real-Time Drug Surveillance Portal under CDSCO remains pending since 2023, limiting data-sharing between laboratories and enforcement units.
- Absence of Dedicated Paediatric Pharmacovigilance: Existing laws (Pre-Conception and Pre-Natal Diagnostic Techniques Act, National Policy for Children 2013) do not address medicine safety for children specifically; Article 39(f) of the Constitution enjoins the State to protect children’s health and development but is insufficiently operationalized in pharmacovigilance.
- Drugs are often only tested for use in adults, with pediatric doses extrapolated from adult data, resulting in unsafe and non-optimized treatments for children.
- There are no pediatric-specific dosage guidelines, leading to errors and adverse drug reactions (ADRs) among children.
- In contrast, the U.S. Pediatric Research Equity Act mandates child-specific studies before marketing approval, ensuring ethical trials and dosage calibration, something absent in India.
- Corruption, Negligence, and Poor Quality Control: A recurring pattern of toxic contaminations shows that the problem is systemic, not accidental.
- In 2022–2023, around 70 childhood deaths in The Gambia and 18 in Uzbekistan were traced to Indian-made syrups.
- Cost-cutting on purification and testing, weak inspections, and corrupt licensing practices sustain such contamination cycles.
- Most state drug control labs lack advanced tools like gas chromatography or mass spectrometers, essential for detecting glycol contamination.
- Over‑the‑Counter (OTC) Misuse and Parental Unawareness: Studies indicate most paediatric medicine purchases in India occur without prescriptions, especially in urban slum areas and Tier‑2 towns.
- Caregivers often double dosing when symptoms persist, unaware that syrup concentrations vary by brand.
- Unregulated sale of paediatric antibiotics and cough syrups drives drug resistance and misuse, worsened by lack of pharmacist education on child-safe dispensing.
- Absence of Comprehensive Paediatric Drug Policy: India still lacks a Paediatric Drug Regulation Authority or dedicated Child-Specific Medicines Code, despite repeated WHO and National Commission for Protection of Child Rights (NCPCR) recommendations.
- Testing for toxins like DEG and EG became mandatory only for export syrups in 2023 after WHO alerts, not for domestic sales.
- WHO officials criticised this “two-tier safety regime” where the global market enjoys better protection than Indian children, calling it a violation of equitable health rights.
- Despite recurring tragedies—from the Jammu (2019) child deaths to the Madhya Pradesh (2025) crisis—no parliamentary white paper has been tabled directly focused on paediatric drug safety.
- Families rarely receive compensation or transparent reporting; most cases are dismissed as “manufacturing defects” without systemic reform.
- Testing for toxins like DEG and EG became mandatory only for export syrups in 2023 after WHO alerts, not for domestic sales.
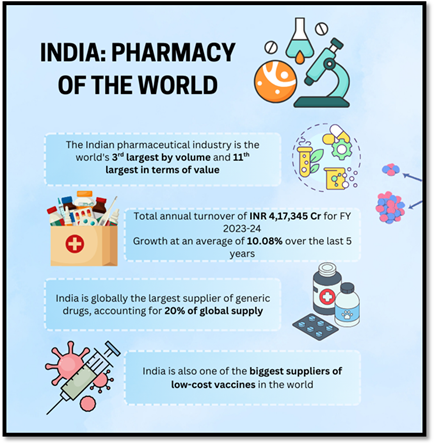
What are the Government Initiatives to Boost the Pharmaceutical Sector in India?
- Production Linked Incentive (PLI) Schemes: The Department of Pharmaceuticals administers three critical PLI schemes.
- PLI Scheme for Pharmaceuticals: Approved in February 2021, with a financial outlay of ₹15,000 crore for FY 2022-23 to FY 2027-28.
- It incentivises 55 selected applicants to manufacture high-value pharmaceutical products, including patented/off-patented drugs, biopharmaceuticals, and complex generics.
- PLI Scheme for KSMs, DIs, and APIs: Launched in March 2020, with an outlay of ₹6,940 crore for FY 2020-21 to FY 2029-30.
- It aims to promote domestic manufacturing of 41 critical Key Starting Materials (KSMs), Drug Intermediates (DIs), and Active Pharmaceutical Ingredients (APIs) to reduce high import dependence.
- PLI Scheme for Medical Devices: Established with a ₹3,420 crore outlay for FY 2020-21 to FY 2027-28.
- It provides financial incentives of 5% on incremental sales of covered medical devices produced in India, supporting cutting-edge manufacturing in sectors such as radiology, imaging, cancer care, and implants.
- PLI Scheme for Pharmaceuticals: Approved in February 2021, with a financial outlay of ₹15,000 crore for FY 2022-23 to FY 2027-28.
- Promotion of Bulk Drug Parks: Approved in March 2020, this scheme supports the development of bulk drug parks with world-class infrastructure to reduce manufacturing costs and strengthen self-reliance.
- Parks in Gujarat, Himachal Pradesh, and Andhra Pradesh were sanctioned, with financial aid capped at ₹1,000 crore per park or 70% of project cost (90% for Northeastern and Hilly States), for a total scheme outlay of ₹3,000 crore.
- Pradhan Mantri Bhartiya Janaushadhi Pariyojana (PMBJP): The PMBJP aims to provide quality generic medicines at affordable prices through a nationwide network of Jan Aushadhi Kendras.
- Strengthening of Pharmaceuticals Industry Scheme (SPI Scheme): A Central Sector Scheme with an outlay of ₹500 crore for FY 2021-22 to FY 2025-26, the SPI scheme aims to support the pharmaceutical industry's infrastructure, capacity building, and innovation.
- Foreign Direct Investment (FDI) Facilitation: In FY 2024-25 (April–December 2024), FDI inflows in pharmaceuticals and medical devices reached ₹11,888 crore.
- Thirteen brownfield FDI proposals worth ₹7,246.40 crore were approved during this period, fostering technological advancement and global competitiveness.
- Atmanirbhar Bharat and Make in India Mission: These missions emphasise indigenous manufacturing to achieve self-reliance, reduce import dependency, and position India as a global hub for pharmaceutical exports.
What Steps Should be Taken to Strengthen the Regulatory Framework of Paediatric Medicines in India?
- Establish a National Paediatric Drug Safety and Ethics Authority (NPSEA): India should create NPSEA under the Central Drugs Standard Control Organisation (CDSCO) to oversee paediatric drug licensing, formulation approval, and ethical trials.
- For instance, the European Medicines Agency (EMA) operates a Paediatric Committee (PDCO), ensuring all new drugs are tested for paediatric use before approval.
- The Mashelkar Committee Report (2003) highlighted the inadequacy of India’s drug regulatory infrastructure and called for greater budgetary allocation to establish a robust, “world-class” drug controlling authority.
- Zero Tolerance Regime and Criminal Accountability:Amend Drugs and Cosmetics Act (1940) to include non-bailable offences for deaths caused by substandard paediatric medicines.
- Introduce mandatory recall and disclosure policy, with real-time updates to public health portals.
- The U.S. Drug Quality and Security Act (DQSA) 2013 created enforceable penalties and a national recall system—India could adopt similar mandates.
- Introduce periodic, unannounced inspections, digital audits, and whistleblower protection for lab staff.
- Create a National Paediatric Clinical Research and Data Ecosystem: Establish a national registry for Paediatric Clinical Trials (PCT-India) to collect pharmacodynamic and safety data.
- Offer fiscal incentives to pharma companies undertaking ethical, India-specific paediatric R&D.
- The U.S. Best Pharmaceuticals for Children Act (BPCA) and Pediatric Research Equity Act (PREA) provide federal funding and data access for drugs studied in children.
- Institutionalize Pharmacist and Caregiver Education:Introduce mandatory certification in paediatric dispensing protocols for pharmacists under the Pharmacy Council of India.
- Run nationwide caregiver awareness drives on dosing and label reading through schools and Anganwadi centers.
- WHO’s “Make Medicines Child Size” campaign (2007) improved medication literacy and pharmacist training in 40+ developing nations.
- Mandatory Batch Testing and Supply Chain Traceability: The approving agencies should ensure compulsory multi-lab toxicological testing (CDSCO + NABL-accredited labs) for all paediatric formulations before release.
- The government should implement QR-based batch tracking to trace product origin to the raw chemical stage.
- The US FDA’s Safe Import Alert System uses digitised testing certification, adaptable for India’s drug traceability.
- International Benchmarking and Ethical Transparency: India’s global pharmaceutical role demands transparency aligned with WHO standards and UNCRC (Article 24).
- India should commit to international drug safety frameworks such as OECD pharmacovigilance standards and ICH E11(R1) guidelines for paediatric medicine development.
- The corrective steps require public disclosure of inspection reports, toxicology results, and recall information to rebuild public trust.
- The European Medicines Agency (EMA) publicly lists all compliance inspection outcomes; India could adopt similar practices.
- Institutional openness will foster accountability, deter negligence, and strengthen India’s reputation as a global pharma hub.
Conclusion:
As the WHO emphasized, “Every child deserves safe, quality healthcare from the very beginning.” To realize this vision, India’s pharmaceutical governance must prioritize ethical regulation, rigorous testing, effective federal coordination, and transparent accountability. By adopting WHO-aligned quality standards, strengthening pharmacovigilance systems, and designing paediatric-focused drug policies, India can evolve from a reactive framework to a proactive guardian of children’s health rights.
|
Drishti Mains Question: From dignity to safety, child health lies at the heart of sustainable development. Critically evaluate whether current policy frameworks sufficiently address paediatric drug safety as a public health priority. |
Frequently Asked Questions (FAQs)
1. What constitutional provisions safeguard children’s health in India?
Articles 21, 39(f), and 47 collectively mandate the State to ensure right to health, nutrition, and protection from neglect, forming the constitutional basis for paediatric healthcare.
2. Which institutions regulate drug quality and pharmacovigilance in India?
The CDSCO oversees approvals and safety; IPC sets quality standards through the Indian Pharmacopoeia; and PvPI monitors adverse drug reactions (ADRs) nationwide.
3. What key challenges impede paediatric drug safety in India?
Weak regulatory coordination, absence of child-specific pharmacovigilance, adult-based dosing, poor lab infrastructure, and OTC misuse compromise medicine safety for children.
4. What government initiatives aim to strengthen the pharmaceutical sector?
Schemes such as PLI for Pharmaceuticals, Bulk Drug Parks, PMBJP, SPI Scheme, and FDI facilitation promote domestic manufacturing, self-reliance, and affordable access.
5. What reforms are proposed to ensure paediatric drug safety in India?
Creation of a National Paediatric Drug Safety Authority (NPSEA), zero-tolerance accountability, mandatory batch testing, paediatric R&D incentives, and WHO-aligned transparency are key measures.
UPSC Civil Services Examination Previous Year Question (PYQ)
Prelims
Q.Which of the following is/are included in the Directive Principles of State Policy? (2008)
1. Prohibition of traffic in human beings and forced labour
2. Prohibition of consumption except for medicinal purposes of intoxicating drinks and of other drugs which are injurious to health
Select the correct answer using the code given below:
(a) 1 only
(b) 2 only
(c) Both 1 and 2
(d) Neither 1 nor 2
Ans: (b)
Mains
Q.What do you understand by Fixed Dose Drug Combinations (FDCs)? Discuss their merits and demerits. (2013)
Q.Can overuse and free availability of antibiotics without doctor’s prescription be contributors to the emergence of drug-resistant diseases in India? What are the available mechanisms for monitoring and control? Critically discuss the various issues involved. (2014)