Governance
Evergreening of Patent
- 27 Mar 2023
- 9 min read
For Prelims: Indian Patent Office, Bedaquiline, Indian Patents Act 1970, Supreme Court.
For mains: Evergreening of Patent.
Why in News?
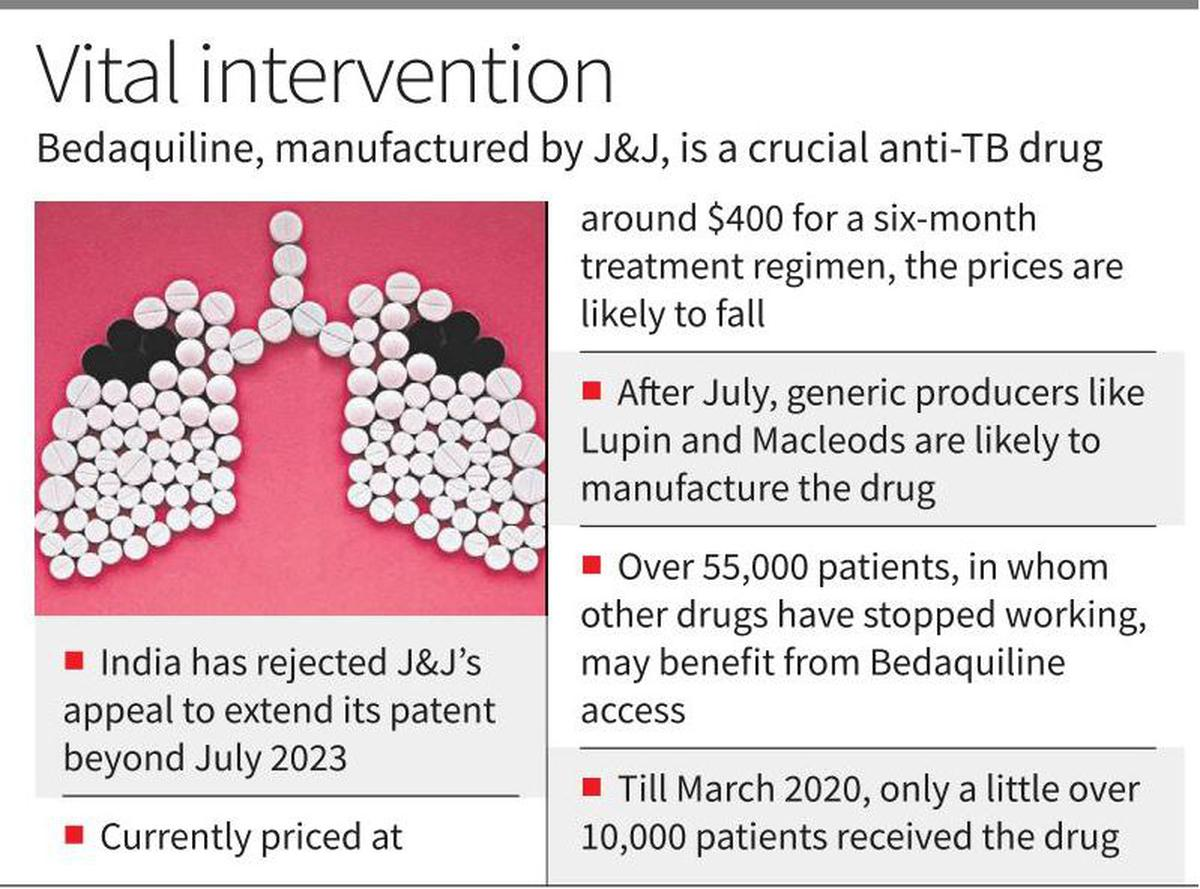
Recently, the Indian Patent Office rejected U.S. pharmaceutical giant Johnson & Johnson’s (J&J) attempt for Evergreening of Patent on manufacturing of the anti-tuberculosis drug Bedaquiline in India beyond July 2023.
- Bedaquiline is a crucial drug in the treatment of multidrug resistant TB patients for whom the first-line drug treatment — using Isoniazid, Rifampicin, Pyrazinamide and Ethambutol — has stopped working.
Why was the Patent Application Rejected?
- J&J’s patent application was for a fumarate salt of a compound to produce bedaquiline tablets.
- It was argued that J&J’s method to produce a “solid pharmaceutical composition” of bedaquiline doesn’t require an “inventive step”.
- According to the Indian Patent Act 1970 Section 2(1) (ja), an ‘inventive step’ is an invention that is “not obvious to a person skilled in the art”.
- The current application drew significantly from a previous patent, which discussed a similar compound on which bedaquiline is based.
- The Patents Act, 1970 has imposed certain ‘restrictions’ on patentability.
- A patent cannot be granted on ‘mere use of a known process, machine or apparatus unless such known process results in a new product or employs at least one new reactant’.
- Section 3(d) of the Act does not allow ‘evergreening’ of patents to prevent innovator pharma companies from extending the patent beyond the stipulated period of 20 years, to ensure that the monopoly does not extend forever.
- As of now it is still a patented product and there are no generic versions. However, after the expiry of the Bedaquiline patent, the drug makers can make the generic versions per the law.
What is Multi Drug Resistant TB (MDR-TB)?
- MDR-TB is a type of tuberculosis infection caused by bacteria that are resistant to at least two of the most effective first-line drugs for TB treatment, isoniazid and rifampicin.
- MDR-TB is caused by Mycobacterium tuberculosis, the same bacterium that causes regular TB, but is much more difficult to treat.
- MDR-TB develops when the bacteria that cause TB mutate and become resistant to the standard drugs used to treat the disease.
- This can occur when patients with regular TB fail to complete their full course of treatment, leading to incomplete eradication of the bacteria and providing the opportunity for the bacteria to develop resistance to the drugs used.
Why is the Rejection Notable?
- The rejection is expected to lower the cost of bedaquiline by up to 80%.
- India has the largest population of people living with drug-resistant TB. J&J’s patent on bedaquiline meant the drug cost USD 400 (revised to USD 340 in 2020) per person, plus the cost of other drugs.
- So far, the Indian government has directly procured the drug and distributed it through State-level TB programmes. After July 2023, manufacturers of generic drugs in India will be able to produce generic versions of bedaquiline.
What is the Evergreening of Patents?
- About:
- The evergreening of patents is a practice of tweaking drugs in order to extend their patent term and thus their profitability.
- The Indian Patents Act 1970 introduced many provisions to prevent the mischievous practice of “evergreening” of patents.
- This is to aid millions of people who can't afford the expensive modified drugs, as well as the development of the domestic generic drug market.
- Concerns:
- The process does not produce any increase in the therapeutic efficacy of the drug. In many countries, minor reformulations can qualify for patent protection. The result is that it prevents competition in the market and is considered harmful to the market and consumers.
- Companies extend the term of protection and charge more for drugs while defending costs incurred in research and development as no cost has been incurred for such compositions as it is only a minor combination or modification of existing drugs.
- Due to the lack of generic drugs, the variety translates into an increase in the cost of healthcare for consumers.
- The persistence of patents primarily affects consumers in underdeveloped and developing countries who cannot afford the brand name drugs that can save them from deadly diseases.
What is a Related Supreme Court Decision?
- A popular precedent in this regard is Novartis vs Union of India case in which the Supreme Court (SC) rejected an appeal filed by Novartis rejecting the patent and upheld that the beta crystalline form of Imatinib Mesylate was a new form of the known substance i.e., Imatinib Mesylate, wherein the efficacy was well known and rejected the patent.
UPSC Civil Services Examination, Previous Year Question (PYQ)
Prelims
Q. Consider the following statements: (2019)
- According to the Indian Patents Act, a biological process to create a seed can be patented in India.
- In India, there is no Intellectual Property Appellate Board.
- Plant varieties are not eligible to be patented in India.
Which of the statements given above is/are correct?
(a) 1 and 3 only
(b) 2 and 3 only
(c) 3 only
(d) 1, 2 and 3
Ans: (c)
Exp:
- Section 3(J) of Indian Patent Act, excludes from patentability “plants and animals in whole or in any part thereof other than microorganisms, including seeds, varieties, and species, and essentially biological processes for production or propagation of plants and animals”. Hence, statement 1 is not correct.
- The Intellectual Property Appellate Board (IPAB) was constituted in 2003 by the Government of India to hear and resolve the appeals against the decisions of the registrar under the Indian Trademarks Act, 1999 and the Geographical Indications of Goods (Registration and Protection) Act, 1999. Hence, statement 2 is not correct.
- Plant variety protection provides legal protection of a plant variety to a breeder in the form of Plant Breeder’s Rights (PBRs). In India, the Protection of Plant Varieties and Farmers’ Rights (PPVFR) Act, 2001, is a sui generis system that aims to provide for the establishment of an effective system for the protection of plant varieties and the rights of plant breeders and farmers. A sui generis system is an alternative to the patent system. Hence, statement 3 is correct.
- Therefore, option (c) is the correct answer.
Mains
Q. Bringing out the circumstances in 2005 which forced amendment to the section 3(d) in Indian Patent Law, 1970, discuss how it has been utilized by the Supreme Court in its judgement in rejecting Novartis’ patent application for ‘Glivec’. Discuss briefly the pros and cons of the decision. (2013)