Important Facts For Prelims
Early Closure of the Antarctic Ozone Hole
- 05 Dec 2025
- 9 min read
Why in News?
The Antarctic ozone hole closed unusually early in 2025, offering one of the strongest signs yet that the ozone layer is on a steady path to long-term recovery.
What is the Antarctic Ozone Hole?
- About: The Antarctic ozone hole refers to the seasonal thinning of the stratospheric ozone layer over Antarctica during the austral spring (September–November).
- Scientists use the term “ozone hole” for regions where ozone levels fall below 220 Dobson Units (DU), not because ozone disappears entirely, but because concentrations drop far below normal.
- This phenomenon was first detected in the early 1980s, when ground-based and satellite measurements showed dramatic drops in ozone levels over the South Pole.
- Reasons for Ozone Holes Over Antarctica:
- Polar Vortex: During the Antarctic winter, a strong and stable polar vortex forms, trapping air and creating extremely cold temperatures in the stratosphere.
- This isolated air mass prevents mixing with warmer air, allowing conditions ideal for ozone-destroying reactions.
- Polar Stratospheric Clouds (PSCs): The extreme cold enables the formation of PSCs.
- These clouds host chemical reactions that activate chlorine and bromine, mainly released from CFCs (chlorofluorocarbons).
- About 80% of stratospheric chlorine and bromine over Antarctica comes from anthropogenic sources.
- Sunlight in Spring: When sunlight returns in spring, these reactive chemicals rapidly destroy ozone molecules, this leads to a large region of severely reduced ozone, known as the “ozone hole.”
- Polar Vortex: During the Antarctic winter, a strong and stable polar vortex forms, trapping air and creating extremely cold temperatures in the stratosphere.
- Ozone Hole Closure: Ozone hole closure refers to the point each year when ozone levels over Antarctica rise above 220 DU again, marking the end of the seasonal thinning.
- As the Antarctic stratosphere warms after spring, polar clouds fade, ozone production resumes, and winds bring in ozone-rich air, restoring the layer and closing the hole.
- The early 2025 closure signals recovery driven by the Montreal Protocol (which phased out CFCs and other ozone-depleting substances), lower chlorine and bromine levels, and favourable stratospheric conditions.
- Significance of Early 2025 Closure: It signals recovery driven by the Montreal Protocol (which phased out CFCs and other ozone-depleting substances) is effective, and cuts harmful UV exposure.
- Boosts confidence that the ozone layer may return to pre-1980 levels globally around 2040, the Arctic by 2045, and the Antarctic by 2066. ozone recovery cools the stratosphere, potentially strengthening Southern Hemisphere jet streams
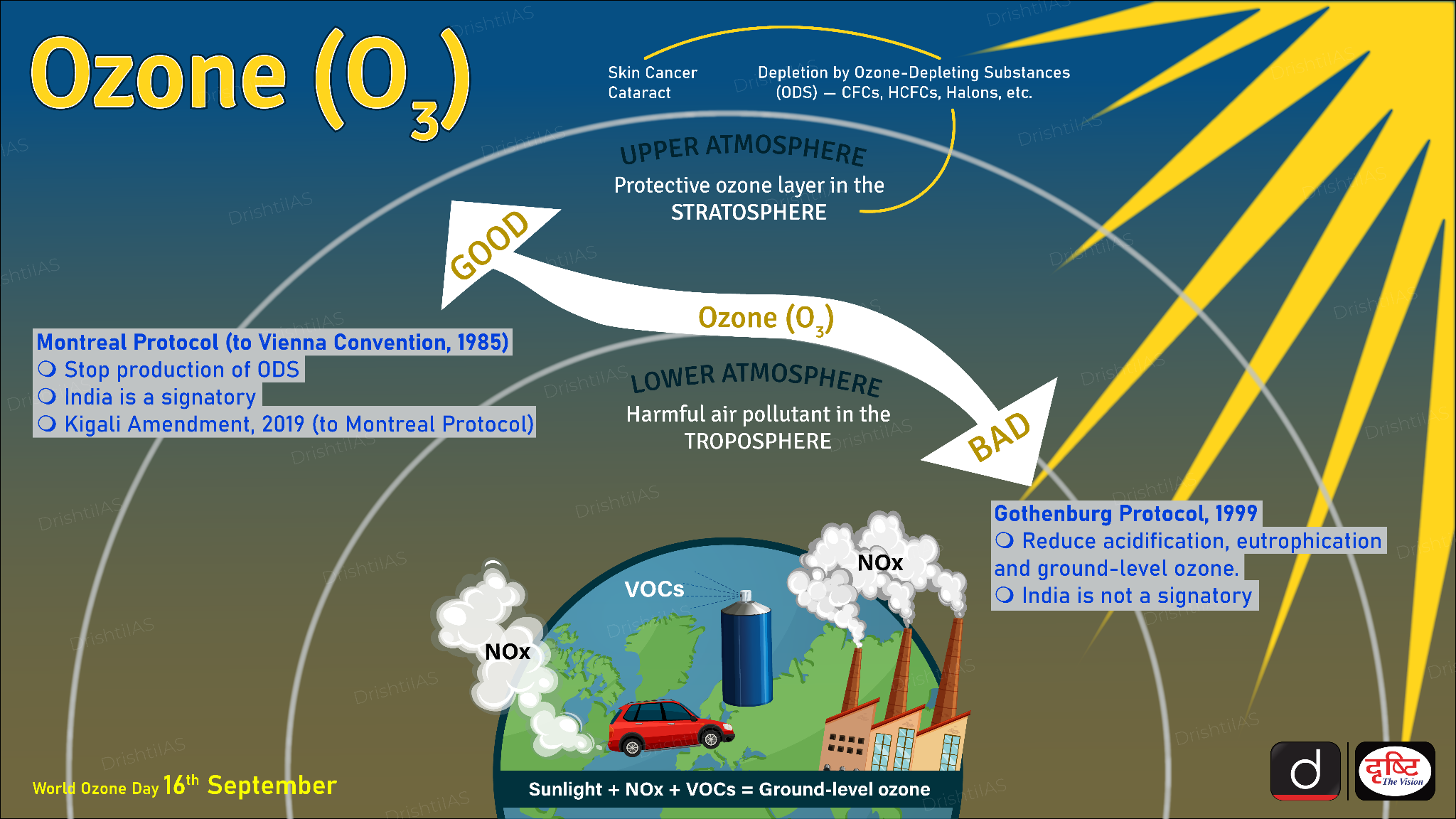
Ozone
- About: Ozone (O₃) is a reactive gas made of three oxygen atoms; it can be natural or man-made.
- It exists in two layers:
- Stratospheric ozone (good ozone): Forms naturally 15–30 km above Earth by the interaction of UV radiation with oxygen. Acts as Earth’s sunscreen by absorbing harmful UV rays.
- Tropospheric ozone (bad ozone): Forms near the ground due to reactions involving Volatile Organic Compounds, and nitrogen oxides, contributing to smog.
- Ozone is measured in Dobson Units (DU); the global average is about 300 DU, with lower values at poles and higher near the equator.
- It exists in two layers:
- Benefits: The ozone layer protects humans and ecosystems by blocking UV radiation that can cause skin cancer, cataracts, immune suppression, and crop and marine damage.
- Depletion: Ozone depletion is driven by chlorine- and bromine-based chemicals such as CFCs, HCFCs, halons, commonly used in refrigeration, air-conditioning, aerosols, foams, and fire extinguishers.
- These chemicals reach the stratosphere where UV light breaks them down, releasing reactive chlorine and bromine that destroy ozone molecules.
- Global Action: The Montreal Protocol (1987) regulates the global phase-out of ozone-depleting substances; it is the first treaty in UN history with universal ratification (2009).
- HFCs, though ozone-safe, are potent greenhouse gases and are being phased down under the Kigali Amendment (2016).
- Phasing out Ozone-Depleting Substances also helps climate mitigation, preventing an estimated 0.5°C to 1°C of warming by 2050.
Frequently Asked Questions (FAQs)
Q. What is meant by the “ozone hole”?
The “ozone hole” denotes regions over Antarctica where column ozone falls below 220 Dobson Units (DU), indicating significant seasonal thinning of stratospheric ozone.
Q. Why did the Antarctic ozone hole close early in 2025?
Early closure in 2025 resulted from a combination of lower stratospheric chlorine and bromine, effective implementation of the Montreal Protocol, and favourable stratospheric temperatures and circulation.
Q. How does the Montreal Protocol contribute to ozone recovery?
The Montreal Protocol (1987) phased out production and consumption of ozone-depleting substances (CFCs, halons, HCFCs), reducing stratospheric chlorine/bromine and enabling gradual ozone layer recovery.
Summary
- The Antarctic ozone hole closed unusually early in 2025, indicating strong ongoing recovery of the ozone layer.
- Ozone depletion occurs when CFC-derived chlorine and bromine react on polar stratospheric clouds and reduce ozone below 220 DU.
- The early closure is driven by the success of the Montreal Protocol, falling ozone-depleting substances, and favourable stratospheric conditions.
- This strengthens expectations that the ozone layer could return to pre-1980 levels by around 2040.
UPSC Civil Services Examination Previous Year Question (PYQ)
Q. Which one of the following is associated with the issue of control and phasing out of the use of ozone depleting substances? (2015)
(a) Bretton Woods Conference
(b) Montreal Protocol
(c) Kyoto Protocol
(d) Nagoya Protocol
Ans: (b)
Q. Consider the following statements: (2012)
Chlorofluorocarbons, known as ozone-depleting substances, are used
- in the production of plastic foams
- in the production of tubeless tyres
- in cleaning certain electronic components
- as pressurizing agents in aerosol cans
Which of the statements given above is/are correct?
(a) 1, 2 and 3 only
(b) 4 only
(c) 1, 3 and 4 only
(d) 1, 2, 3 and 4
Ans: (c)
Q. Consider the following: (2019)
- Carbon monoxide
- Methane
- Ozone
- Sulphur dioxide
Which of the above are released into atmosphere due to the burning of crop/biomass residue?
(a) 1 and 2 only
(b) 2, 3 and 4 only
(c) 1 and 4 only
(d) 1, 2, 3 and 4
Ans: (d)