Important Facts For Prelims
Detection of Barium in the Exoplanet Atmospheres
- 15 Oct 2022
- 3 min read
Why in News?
Recently, in a new study, scientists have detected barium in the upper atmosphere of two giant exoplanets for the first time.
- Ultra-hot Jupiters are a class of hot gaseous planets that matches the size of Jupiter, but they have short orbital periods, unlike Jupiter.
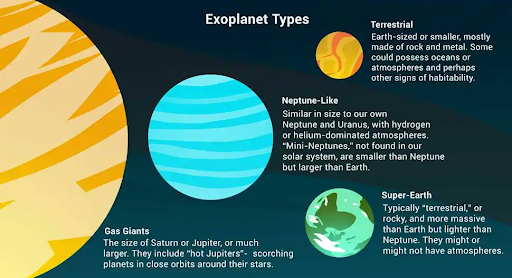
What are Exoplanets?
- An exoplanet or extrasolar planet is a planet outside the Solar System. The first confirmation of detection of exoplanets occurred in 1992.
- More than 4,400 exoplanets have been discovered till now.
- They are very hard to see directly with telescopes. They are hidden by the bright glare of the stars they orbit. So, astronomers use other ways to detect and study exoplanets such as looking at the effects these planets have on the stars they orbit.
What are the Findings of the Study?
- The exoplanets are two ultra-hot Jupiters — WASP-76b and WASP-121b — which orbit their host stars WASP 76 and WASP 121.
- The former is about 640 light-years away from the Earth and the latter around 900 light-years away.
- Both WASP-76b and WASP-121b complete one orbit in two days.
- Surface temperatures in these bodies reach as high as 1,000 degrees Celsius. These bodies have unique features owing to their high temperatures. For instance, WASP-76b experiences iron rain.
- The presence of hydrogen, lithium, sodium, magnesium, calcium, vanadium, chromium, manganese and iron in the atmosphere of the WASP-76 b has also been confirmed in addition to barium.
- In WASP 121b, they confirmed the presence of lithium, sodium, magnesium, calcium, vanadium, chromium, manganese, iron and nickel.
- Additionally, the team found elements such as cobalt and strontium. They also found indications of titanium in the exoplanet.
What are the Characteristics of Barium?
- About:
- Barium, which is slightly harder than lead, has a silvery white luster when freshly cut.
- It readily oxidizes when exposed to air and must be protected from oxygen during storage.
- In nature it is always found combined with other elements.
- It is very light and its density is half of that of iron.
- Uses:
- Barium is often used for spark-plug electrodes and in vacuum tubes as a drying and oxygen-removing agent. As well as fluorescent lamps: impure barium sulfide phosphorescence after exposure to light.
- Its compounds are used by oil and gas industries to make drilling mud. Drilling mud simplifies drilling through rocks by lubricating the drill.
- Barium compounds are also used to make paint, bricks, tiles, glass, and rubber.
- Barium nitrate and chlorate give fireworks a green colour.