Science & Technology
Electric Batteries and Electrochemical Cells
- 16 Nov 2023
- 7 min read
For Prelims: Electrochemical Cells, Electric vehicles, Voltaic Cells, Lithium-ion Technology, Nobel Prize in Chemistry for 2019, Applications of Electric Batteries.
For Mains: Electrochemical Cells, Evolutionary Trajectory of Batteries.
Why in News?
The electric batteries and electrochemical cell advancements have garnered significant attention for revolutionizing technology across sectors like transport and energy, steering us toward a sustainable future.
What are Electric Batteries and Electrochemical Cells?
- Electric Batteries:
- An Electric battery is a device that stores chemical energy and converts it into electricity.
- Batteries are made up of one or more electrochemical cells that are connected to external inputs and outputs.
- Electric batteries have transformed our world, enabling the proliferation of motorization and wireless technology.
- Major Applications:
- Portable Electronics: Powering smartphones, laptops, tablets, and wearable devices.
- Transportation: Driving electric vehicles (EVs) for both personal and public transportation, reducing reliance on fossil fuels.
- Renewable Energy Storage: Storing energy generated by solar panels and wind turbines for later use.
- Electricity for Remote Areas: Providing electricity in remote or off-grid locations where conventional power sources are unavailable or unreliable.
- An Electric battery is a device that stores chemical energy and converts it into electricity.
- Major Types of Batteries:
- Solid-state battery: It is a battery that uses solid electrodes and a solid electrolyte instead of a liquid or polymer gel electrolyte.
- Solid-state batteries are used in a variety of devices, including: pacemakers, radio frequency identifications (RFID) and wearable devices.
- Nickel–Cadmium battery (Ni-Cd): They are used for Cordless electronic appliances, drills, camcorders and other small battery-operated devices requiring an even power discharge.
- Alkaline Battery: This is a type of primary battery that uses zinc and manganese dioxide as electrodes.
- It is used for applications that require low cost and reliable power, such as flashlights, toys, radios, and remote controls.
- Lithium-ion Battery: The Li-ion battery's groundbreaking principles earned its developers the Nobel Prize in Chemistry in 2019, underscoring its profound impact in the 20th and 21st centuries.
- Li-ion batteries are versatile, powering portable devices like phones and laptops as well as fueling electric vehicles such as cars and bikes.
- Solid-state battery: It is a battery that uses solid electrodes and a solid electrolyte instead of a liquid or polymer gel electrolyte.
- Electrochemical Cells:
- Electrochemical cells are devices that can convert chemical energy into electrical energy, or vice versa.
- They can produce an electric current through chemical reactions, or they can use electrical energy to facilitate chemical reactions.
- Electrochemical cells, like voltaic or galvanic cells, operate via redox reactions wherein electrons are liberated during oxidation and utilized during reduction.
- A standard cell comprises two sections accommodating metal electrodes immersed in specific electrolytes.
- The electrodes, namely the anode and the cathode, conduct electricity.
- The anode, where oxidation occurs, and the cathode, where reduction takes place, form the fundamental components of the cell.
- A standard cell comprises two sections accommodating metal electrodes immersed in specific electrolytes.
- Electrons flow from the negatively charged anode to the positively charged cathode through an external circuit, providing power for a variety of uses.
- Connecting these halves is a wire and a salt bridge, facilitating the movement of ions between them.
- The energy carried by electrons dictates the source voltage, steering the electron flow within the circuit.
- In ideal conditions, the source voltage is equal to the terminal voltage, ensuring an efficient power supply.
- Advancements in cell design and materials, seen in nickel-cadmium, zinc-copper, and modern lithium-ion cells, showcase increased voltages and enhanced efficiency.
- Electrochemical cells are devices that can convert chemical energy into electrical energy, or vice versa.
- Related Challenges:
- One of the well-known challenges affecting the efficiency of electrochemical cells is corrosion. For instance, in environments with high humidity, electrodes can gather water droplets.
- If the atmospheric carbon dioxide levels are elevated, the combination of water and gas leads to the formation of carbonic acid, causing corrosion on the electrode surfaces.
- Another issue arises from galvanic corrosion, where one of the electrodes within a cell deteriorates faster in the electrolyte due to its higher reactivity.
- For instance, in a carbon-zinc battery, the zinc electrode erodes more rapidly during the battery's usage.
- One of the well-known challenges affecting the efficiency of electrochemical cells is corrosion. For instance, in environments with high humidity, electrodes can gather water droplets.
What is the Evolutionary Trajectory of Batteries?
- Galvani's Experimentation (1780):
- Luigi Galvani's experiment involving metals and frog legs revealed a curious connection between electrical energy and muscle movement, laying the groundwork for future battery development.
- Voltaic Pile (1800):
- Alessandro Volta's voltaic pile marked a significant step, generating a steady current using metal plates and electrolytes.
- However, its functionality remained a mystery.
- Alessandro Volta's voltaic pile marked a significant step, generating a steady current using metal plates and electrolytes.
- Faraday's Insights (Early 19th Century):
- Michael Faraday's groundbreaking work deciphered the mechanisms behind the cells, unveiling the roles of components like anode, cathode, and electrolyte.
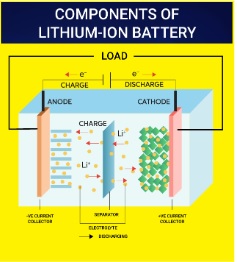
- Lithium-Ion Batteries: This battery functions as both a voltaic and an electrolytic cell, capable of converting chemical energy into electrical energy and vice versa, enabling recharging.
- In lithium-ion cells, lithium metal oxide and graphite act as cathode and anode, respectively, with a semisolid polymer gel electrolyte separating them.
- The intercalation process enables charge and discharge phases.
Note: The Nobel Prize in Chemistry for 2019 was awarded to John B. Goodenough, M. Stanley Whittingham, and Akira Yoshino for their contributions to the development of the lithium-ion battery.
UPSC Civil Services Examination, Previous Year Question (PYQ)
Q. Which one of the following pairs of metals constitutes the lightest metal and the heaviest metal, respectively? (2008)
(a) Lithium and mercury
(b) Lithium and osmium
(c) Aluminium and osmium
(d) Aluminium and mercury
Ans: (b)