Technologies Shaping the Pharma Industry | 02 Jun 2025
For Prelims: Pharmaceutical, Generic Drug, Biotechnology Sector, Active Pharmaceutical Ingredients (APIs), Production-Linked Incentive (PLI) Scheme, Promotion of Bulk Drug Parks Scheme, mRNA Vaccine. Blockchain technology
For Mains: Technologies Shaping Pharma Industry, State of Pharma Industry in India, Opportunities and Challenges in India’s pharmaceutical industry.
Why in News?
The pharmaceutical industry is evolving rapidly with biologics, AI, and automation driving changes in drug development and manufacturing. To stay competitive globally, India must foster specialized skills in these technologies and address key challenges like regulatory compliance, infrastructure, and innovation capacity.
What are the Major Technologies Shaping the Pharmaceutical Industry?
- Artificial Intelligence (AI) and Machine Learning (ML): AI and ML speed up drug discovery by predicting molecular behavior, identifying new uses for existing medicines, and personalizing treatments.
- Generative AI, especially Large Language Models LLMs), enhances understanding of biology and helps design more effective clinical trials using data such as genetics.
- In India, the Centre for AI and Robotics (CAIR) under DRDO, is actively developing AI applications that can also be beneficial for the pharmaceutical sector.
- In India, leading pharmaceutical companies like Sun Pharma and Dr. Reddy's Laboratories are deploying AI to tackle diseases with high national burdens, such as tuberculosis and diabetes.
- Internet of Medical Things (IoMT): IoMT integrates IoT devices and mobile apps to monitor health parameters like heart rate, blood pressure, and glucose levels in real time.
- It enables personalized treatment and supports decentralized clinical trials (DCTs), enhancing patient access, convenience, and trial efficiency.
- IoT-enabled packaging monitors storage conditions like temperature and light exposure, ensuring compliance with regulatory standards and preventing spoilage.
- Blockchain for Data Transparency: Blockchain technology ensures privacy, transparency, and traceability in the pharmaceutical supply chain.
- It enables secure access to medical records, accredits suppliers, tracks drug prices, and helps detect counterfeit or substandard medicines, improving regulatory compliance and patient safety.
- For instance, Indian Institute of Technology Madras researchers have developed 'BlockTrack', a first-of-its-kind blockchain-based secure medical data and information exchange.
- Biologics and Biosimilars: Biologics are complex medicines derived from living organisms, such as vaccines, monoclonal antibodies, recombinant proteins, and cell therapies.
- Biosimilars are cost-effective, clinically equivalent versions of biologics developed after patent expiry.
- Organ bioprinting uses 3D printing technology to create living, functional organs from bioinks containing cells and other biomaterials.
- Biocon is a leading Indian biotech company working on biosimilars and insulin products.
- Digital Twin Technology: Digital Twin Technology uses real-time data to create virtual simulations of physical processes.
- In pharmaceuticals, it helps simulate drug production lines to improve manufacturing efficiency, reduce downtime, and optimize operations.
What is the State of Pharmaceutical Industry in India?
- About: India ranks as the world’s 3rd-largest producer of pharmaceuticals by volume and stands 14th globally by value.
- It supplies over 50% of the global vaccine demand and nearly 40% of generic medicines in the US market.
- Market Size: For FY 2023-24, India’s pharmaceutical market is valued at approximately USD 50 billion, contributing about 1.72% to the national GDP.
- It is projected to grow to USD 130 billion by 2030. India’s biotechnology sector, valued at USD 137 billion in 2022, aims to reach USD 300 billion by 2030.
- Key Segments:
- Generic Medicines: India is the world’s largest supplier, fulfilling 20% of global demand.
- Active Pharmaceutical Ingredients (APIs): India manufactures over 500 APIs, accounting for 8% of the global API market.
- Medical Devices: The sector is expected to expand from USD 11 billion to USD 50 billion by 2030.
- Growth Drivers:
- Affordable Pricing: Indian pharmaceuticals are significantly more cost-effective compared to Western counterparts.
- Government Support: Initiatives like the Production-Linked Incentive (PLI) scheme encourage domestic manufacturing.
- Robust R&D: India boasts a strong scientific and engineering workforce, ranking 6th globally in patent filings with 64,480 patent applications in 2023.
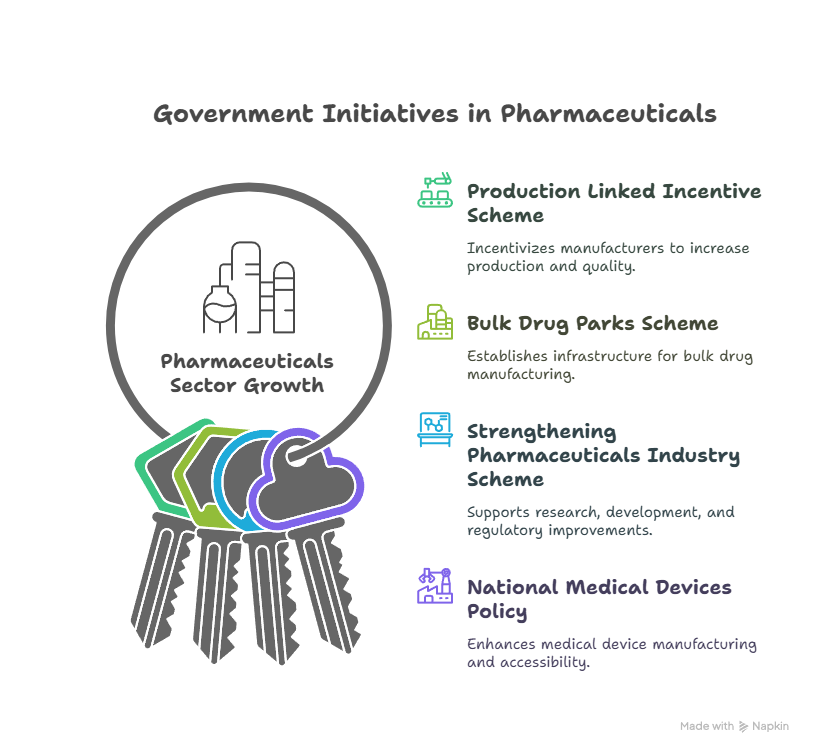
- Related Government Initiatives:
What are the Key Concerns Associated with Recent Technological Breakthroughs in the Pharma Sector?
- Data Privacy & Cybersecurity Challenges: The use of AI, big data analytics, and cloud systems in pharma has led to a surge in sensitive patient and clinical data. This raises serious concerns about data privacy, cybersecurity risks, and potential breaches, which may compromise patient confidentiality and public trust in healthcare systems.
- Escalating Costs & Barriers to Access: Technologies like biologics, AI platforms, and automation need heavy investment in infrastructure, equipment, and skilled workforce. This high cost burdens small and medium enterprises (SMEs), widening the gap with larger firms and limiting the affordable and widespread adoption of innovations across the pharma sector.
- Regulatory Complexities and Delays: Rapid technological progress in pharma often outpaces regulatory reforms, making it challenging to ensure both patient safety and swift approvals. The absence of clear and globally harmonized guidelines leads to confusion and delays in bringing new therapies and innovations to market.
- Skill Deficits & Workforce Preparedness: The adoption of AI, biotech, and automation in pharma demands interdisciplinary skills. However, India faces a major talent gap in areas like data science, bioinformatics, and robotics, which hampers effective implementation of these technologies.
- Ethical, Social, & Equity Concerns: Innovations like gene editing, AI diagnostics, and personalized medicine raise ethical issues around consent, data bias, and fair access. These challenges call for strong ethical frameworks to prevent societal harm and ensure inclusive healthcare delivery.
What Measures can be Adopted to Ensure Responsible Technological Intervention in the Pharma Sector?
- Robust and Adaptive Regulatory Ecosystems: Regulatory frameworks must be agile and forward-looking, enabling faster approvals while ensuring patient safety. Adaptive regulations can balance innovation with efficacy validation.
- Strengthening Data Privacy, Security, & Ethics: Pharma firms should adopt end-to-end encryption, blockchain for traceability, and AI-based threat detection as per the Digital Data Protection Act, 2023. Ethical AI frameworks are essential to reduce bias and uphold fairness.
- Investment in Human Capital & Digital Skills: Collaboration between government, academia, and industry should focus on reskilling programs, digital literacy, innovation labs, and mentorship platforms to build a future-ready workforce.
- Institutionalizing Ethical and Social Responsibility: Independent ethics committees, transparent clinical trial protocols, and inclusive public engagement are key to managing concerns around consent, equity, and long-term impact of technologies like gene editing and AI diagnostics.
- Promoting Collaborative & Open Innovation: Public-private partnerships (PPPs), open-source platforms, and global consortia can pool knowledge, share risks, and accelerate innovation in a cost-effective and inclusive manner.
Conclusion
Bridging the skill gap in emerging technologies like AI, biotechnology, and automation is crucial for sustaining India’s leadership in the pharmaceutical sector. Strengthening these capabilities will not only enhance drug discovery and manufacturing but also ensure India’s competitiveness in the global pharma landscape amid rapid technological transformation.
|
Click Here to Read More: Pharmaceutical Regulation in India, Challenges Related to the Pharma Industry |
|
Drishti Mains Question Discuss the current status and global significance of the Indian pharmaceutical industry. How have recent technological advancements impacted its growth and competitiveness? |
UPSC Civil Services Examination, Previous Year Question (PYQ)
Prelims
Q. Which of the following are the reasons for the occurrence of multi-drug resistance in microbial pathogens in India? (2019)
- Genetic predisposition of some people
- Taking incorrect doses of antibiotics to cure diseases
- Using antibiotics in livestock farming
- Multiple chronic diseases in some people
Select the correct answer using the code given below.
(a) 1 and 2
(b) 2 and 3 only
(c) 1, 3 and 4
(d) 2, 3 and 4
Ans: (b)
Mains
Q. How is the Government of India protecting traditional knowledge of medicine from patenting by pharmaceutical companies? (2019)