Draft Patent Amendment Rules Undermine Pre-grant Opposition | 06 Oct 2023
For Prelims: Patent, Evergreening of Patents, Pre-grant Opposition
For Mains: Impact of Patent rules on the Production and Availability of Affordable Generic Drugs, Intellectual Property Rights (IPRs) .
Why in News?
Recent draft patent amendment rules in India proposed by the Department for Promotion of Industry and Internal Trade (DPIIT) have raised concerns over their potential impact on affordable drugs and vaccines. These rules may hinder pre-grant opposition, a vital safeguard against unwarranted patent extensions, posing challenges to public health.
What are the Draft Patent Amendment Rules?
- Draft Patent Amendment Rules
- About:
- The draft patent amendment rules are a set of proposed modifications to the existing patent rules in India, which regulate the procedures and fees for filing, examining, granting, and opposing patents.
- Main Features:
- The introduction of variable fees for filing pre-grant oppositions, which could range from Rs. 1,500 to often exceeding Rs. 40,000, depending on the category and number of applicants, has been implemented.
- The provision of granting the controller of patents the power to determine the maintainability of representation by individuals or civil society organizations seeking to file pre-grant oppositions.
- The increase of the official fee for filing post-grant oppositions, which will be equal to the aggregate patent filing cost incurred by the applicant.
- About:
- Concerns:
- Restricting Access to Affordable Drugs:
- The proposed rules may limit access to affordable generic drugs, by making it harder to challenge patents.
- The introduction of variable fees for filing pre-grant oppositions could impose a significant financial burden on civil society organizations and patient groups.
- Controller's Discretion:
- Under the current Patents Act, 1970 any person can file a pre-grant opposition, providing a democratic approach to challenging patents.
- However, the draft rules propose to give the controller the authority to decide the maintainability of those filing pre-grant oppositions. This shift in power has raised concerns about potential biases and challenges for those seeking to oppose patents.
- Under the current Patents Act, 1970 any person can file a pre-grant opposition, providing a democratic approach to challenging patents.
- Impact on Public Health Safeguards:
- Pre-grant opposition serves as a crucial public health safeguard against practices like patent evergreening and the granting of unwarranted monopolies.
- Evergreening of patents is a strategy to extend the term of a patent by obtaining new patents before the original one expires. In India, Section 3(d) of the Patent Act 1970 (amended in 2005) prohibits granting patents for new forms of known substances unless they significantly differ in efficacy. Therefore, evergreening is not allowed under Indian patent law.
- It ensures continued accessibility to quality-assured and affordable generic medicines.
- Weakening pre-grant opposition could lead to unwarranted patent extensions, limiting access to essential medicines and vaccines.
- Pre-grant opposition serves as a crucial public health safeguard against practices like patent evergreening and the granting of unwarranted monopolies.
- Pharma Lobbying:
- Concerns have been raised that the rules favour pharmaceutical companies and may undermine India's unique provision of the pre-grant opposition.
- Global Impact:
- The proposed changes could disproportionately impact patients in India and the global South, who rely heavily on India's production of affordable generic drugs and vaccines.
- A threat to access to essential medicines may put patients at risk and affect the generic drug industry.
- The proposed changes could disproportionately impact patients in India and the global South, who rely heavily on India's production of affordable generic drugs and vaccines.
- Restricting Access to Affordable Drugs:
Notable Instances of Successful Pre-grant Oppositions
- Pre-grant oppositions by patient groups and civil society organizations have frequently led to the rejection of patent extensions sought by big pharmaceutical companies based on weak claims of "novel invention."
- Tenofovir Disoproxil Fumarate (TDF):
- In 2006, patient groups contested Sahara's TDF patent due to the drug's use of a known compound.
- Nevirapine:
- Boehringer Ingelheim's pediatric Nevirapine patent was denied in 2008 following a pre-grant opposition, as it failed to show a significant improvement in efficacy.
- Glivec:
- Novartis' cancer drug Glivec faced rejection by the Supreme Court of India in 2013, as it was considered a modified version of an existing drug, Imatinib.
- Tenofovir Disoproxil Fumarate (TDF):
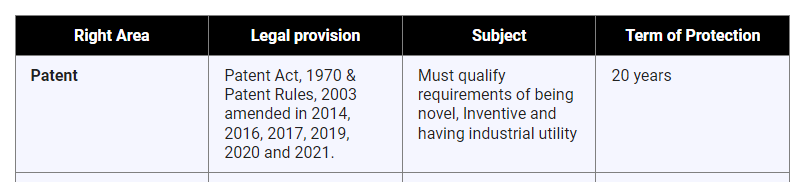
- Patent:
- About:
- A Patent is a statutory right for an invention granted for a limited period of time to the patentee by the Government, in exchange of full disclosure of invention for excluding others, from making, using, selling, importing the patented product or process for producing that product for those purposes without his consent.
- Patent protection is a territorial right and therefore it is effective only within the territory of India. There is no concept of global patent.
- Patentability Criteria for an Invention:
- It should be novel.
- Must involve an inventive step (technical advancement)
- Capable of industrial application.
- About:
- Opposition Against Grant of a Patent :
- The Indian Patent Act, 1970 allows the public to file objections against patents at two stages:Pre-grant opposition and Post-grant opposition.
- Pre-grant opposition:
- Filing an Opposition:
- Any person can file a pre-grant opposition in writing after the patent application's publication but before it's grant. Complete specifications are needed, not just the abstract.
- Grounds for Opposition:
- Wrongful Obtainment(Invention was wrongfully obtained), Prior Publication, Prior Claim, Prior Knowledge or Use, Obviousness, Non-Patentable Subject Matter, Insufficient Description, Non-Disclosure (Failure to disclose required details), False Disclosure, Time Limit(Conventional application not filed within 12 months from the first patent application), Biological Material( Failure to disclose the origin or source), Traditional Knowledge( The invention was anticipated using indigenous community knowledge).
- Filing an Opposition:
- Post-grant opposition:
- Once the patent has been granted, a written opposition can be filed after publication, and it must be submitted to the Controller within 12 months of the patent's publication in the Indian Patent Journal.
- Grounds for opposition are the same as in pre-grant opposition.
UPSC Civil Services Examination, Previous Year Question (PYQ):
Mains:
Q. Bringing out the circumstances in 2005 which forced an amendment to the section 3(d) in Indian Patent Law, 1970, discuss how it has been utilized by the Supreme Court in its judgement in rejecting Novartis’ patent application for ‘Glivec’. Discuss briefly the pros and cons of the decision. (2013)