Biosimilars | 31 Oct 2025
The US Food and Drug Administration (FDA) has released new draft guidelines aimed at reducing the cost and time for biosimilar development. This shift is expected to significantly benefit Indian pharma companies which have invested heavily in biosimilar pipelines.
- This can remove the requirement for comparative efficacy trials, saving USD20–25 million per project, and shorten development timelines from 5–7 years to 3–4 years.
Biosimilar
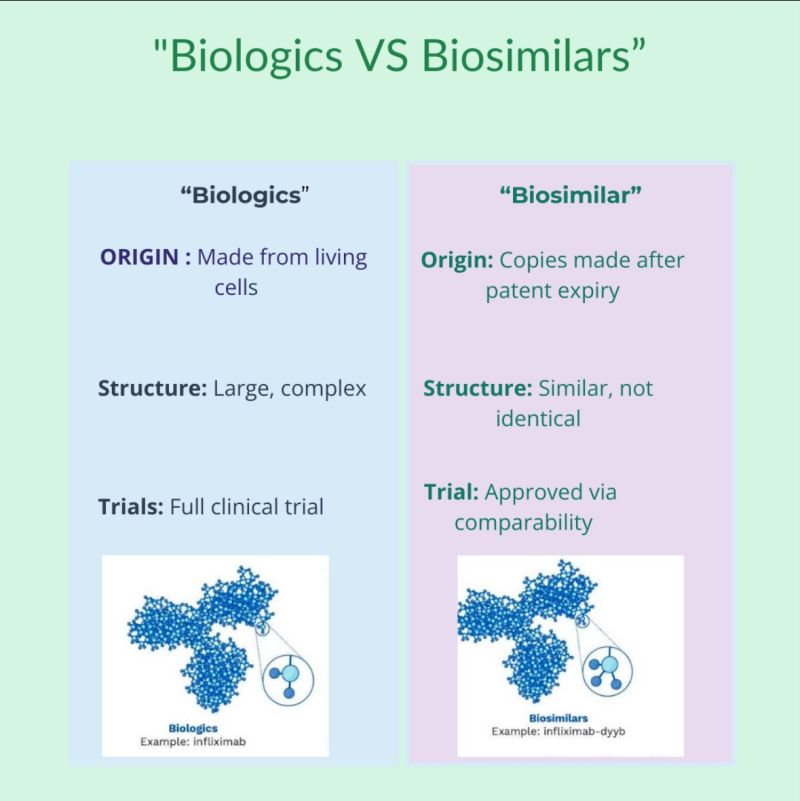
- About: A biosimilar is a biologic medication that is highly similar to an already approved biologic, known as the reference product.
- A biosimilar is not a generic drug; unlike generics, which are simple chemical copies, biosimilars are complex proteins made from living cells, highly similar but not identical to the original biologic.
- Composition: Both biosimilars and their reference biologics are made from living sources such as cells, tissues, microorganisms (bacteria or yeast), or combinations of natural materials like proteins and sugars.
- Cost Advantage: Biosimilars are typically available at a lower cost than the original biologics, improving patient access to affordable treatments.
- India’s Position: Indian pharma firms hold under 5% of the USD 30 billion global biosimilars market, but are expanding through the National Biopharma Mission and Genome Valley expansion in Telangana.
- Indian biosimilar exports, currently valued at USD 0.8 billion, are projected to grow 5x to USD 4.2 billion by 2030, capturing 4% of the global market, and reach USD 30-35 billion by 2047.
- Application: Biosimilars are safe and effective for treating various chronic diseases, including cancer, diabetes, and autoimmune disorders.
| Read More: Technologies Shaping the Pharma Industry |