Medical Devices In India
Introduction
- The medical device industry is a unique blend of engineering and medicine. It involves the creation of machines that are used to support life within the human body.
- Medical devices include Surgical Equipment, Diagnostic equipment like Cardiac imaging, CT scans, X-ray, Molecular Imaging, MRI and Ultrasound-imaging including hand - held devices; Life Support equipment like ventilator, etc. as well as Implants and Disposables.
- Medical devices, unlike pharmaceuticals, are dependent on a mix of technologies such as engineering, electronics, material sciences and information technology.
Medical Devices Sector in India
- Medical devices sector in India is very small in size as compared to the rest of the manufacturing industry, though India is one of the top twenty markets for medical devices in the world and is the 4th largest market in Asia after Japan, China, and South Korea.
- India currently imports 80-90% of medical devices of the $15 billion market, the vast majority of which are unregulated for quality and safety.
Factors Driving the Growth of Medical Devices Sector
- Market Factors– Growing population, ageing, income base and associated disposable income, increasing socio-economic inclusion of rural and deprived in mainstream economy, heightened manufacturing innovation to create customized products to meet the needs of all income segments, changing disease prevalence pattern (e.g. early onset of diabetes and heart diseases) and growing awareness among the middle class to focus on early detection and disease prevention.
- Non-market Factors– Development of infrastructure, favorable regulations, FDI inflow, outsourcing of manufacturing and R&D activities to India, government initiatives to improve healthcare access through insurance schemes such as Rashtriya Swasthya Bima Yojana (RSBY), Aarogyasri, etc.
Issues Regarding Medical Devices Sector in India
- Underdeveloped: The industry is still at a nascent stage with sub-optimal penetration and usage of medical devices. This demands innovation and R&D from the medical device industry in order to push for indigenisation.
- Regulatory Loopholes/deficit: The lack of regulatory systems, harmonized standards, accreditation, legal requirements, proper guidance on quality and best practices etc. are affecting the medical devices industry adversely.
- Medical devices continue to be under the Drugs and Cosmetics Act 1940 and industry representatives are pushing for a comprehensive regulation that views medical devices separately, through the entire life-cycle of the product, from design to tests on patients, incorporating doctor feedback, and surveillance of patients in whom the implants are used, etc.
- The four draft notifications issued by the health ministry recently has notified all medical devices as ‘medicines’ under the Drugs Act from December 2019.
- However, experts and device manufacturers are demanding to bring a separate law, Medical Devices Act, to regulate the medical devices industry in India. At present only 24 out of over 6,000 devices are regulated.
- The Drugs Act itself needs reforms as it does not uniformly and equitably regulate quality from state-to-state in the absence of a national singular regulatory authority. The recent J&J episode showed the limitations of the Drugs Act and the Drugs Controller was seen to be handicapped to discipline overseas manufacturers.
- Presently, India does not have any legal provisions to compensate patients facing health problems due to implants or the use of faulty medical devices. Under the law, companies are liable to pay compensation only when something goes wrong during a clinical trial.
- The Johnson and Johnson’s faulty hip implant device case is symptomatic of the failure of regulatory mechanisms to counter corporate forgery, lack of administrative accountability, breach of business/medical ethics of big pharma, and the lack of consumer/patient awareness.
- Investors shy away from an unpredictable, incomplete and incorrect regulatory environment. In the absence of norms, domestic manufacturing suffers as a surgeon is unsure of trying an unregulated device from a startup on a patient.
Major Recommendations of Task Force on the Medical Devices Sector In India (2015)
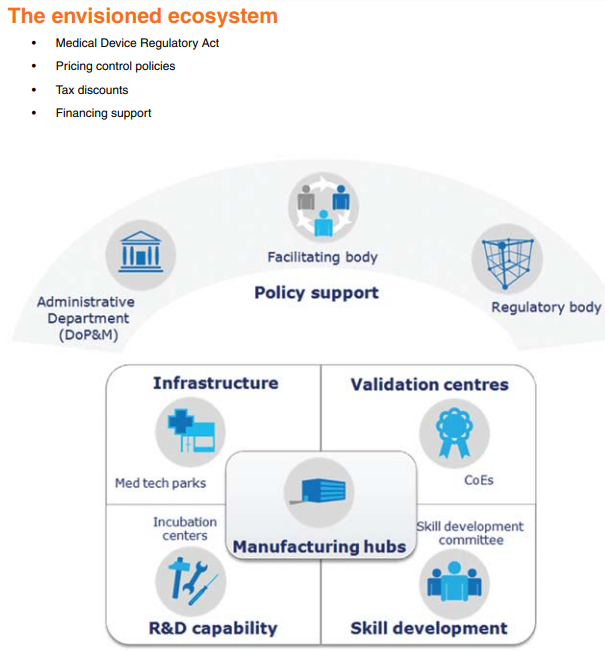
- Formulate “Medical Device Regulatory Act”: Medical devices should be treated distinctly from drugs and a separate chapter for medical devices should be made in the existing Drugs & Cosmetics Act.
- Create necessary bodies to drive the policies: Set up an independent body with a permanent office and support staff to promote and facilitate the medical device industry with representatives from all related government departments as well as Industry.
- Department of Pharmaceuticals should be strengthened and rechristened as the Department of Pharmaceuticals and Medical Devices.
- Preferential treatment in government procurement: Preference may be given to medical devices that are being manufactured in India with an additional preference for medical devices manufactured under the MSME sector.
- Medical device testing centers should be set up preferably in the PPP model.
- Designate “Centers of Excellence” (CoE) for supporting product development and validation.
- Set up a Skill development committee with representatives from the Medical devices industry, academia (NIPERs) and Healthcare Sector Skill Council (HSSC) under the National Skill Development Council (NSDC).
- Separate price control order for medical devices.
Recent Government Steps
- The government has approved setting up four medical device parks with a view to support Make in India initiative and provide world-class treatment at affordable prices. The parks will be set up in Andhra Pradesh, Telangana, Tamil Nadu and Kerala. Uttarakhand and Gujarat have also approached the Centre for such parks.
- These parks will provide the necessary infrastructure, where companies can easily plug and play.
- This will not only cut import bills but will also help in easy access to standard testing facilities and reduce the cost of production.
- Government of India in November 2018 approved formula for determination of compensation for those patients who had received (prior to August 2010) faulty Articular Surface Replacement (ASR) hip implants manufactured by M/s. DePuy International Limited, U.K (M/S Johnson & Johnson Pvt Ltd).
- New provisions will be added to the Drugs and Cosmetics Act to introduce a compensation plan for faulty medical devices having adverse impacts on patients.
Conclusion
- There is a difference between medical devices and drugs so it would be a grave mistake to apply the same regulatory framework for both of them. A targeted and different approach is needed to regulate these complex devices.
- Medical devices sector, being a critical sector dealing with human lives, needs to be given sufficient attention on an urgent basis to make the sector effective and transparent with the aim to make healthcare devices affordable and accessible to the masses.
- India being a large market for medical devices should tap the potential generated by the Medical Devices Industry by strengthening its domestic framework that would greatly help in realising the vision of “Make in India”.
For Mind Map
