Sansad TV Vishesh: CAR T-Cell Therapy | 24 Apr 2024
For Prelims: CAR T-Cell Therapy, Chimeric Antigen Receptor T-cell Therapy, Leukaemias, Lymphomas, Living Drugs, White Blood Cell, NexCAR19, Central Drugs Standard Control Organisation (CDSCO), Chemotherapy, CRISPR-Cas9 , Platelets
For Mains: Significance of CAR T-Cell Therapy in treatment of cancer patients.
Why in News?
Recently, The President of India remarked indigenously developed CAR-T Cell Therapy for the treatment of cancer as a significant success in an event organized at the Indian Institute of Technology (IIT) Bombay.
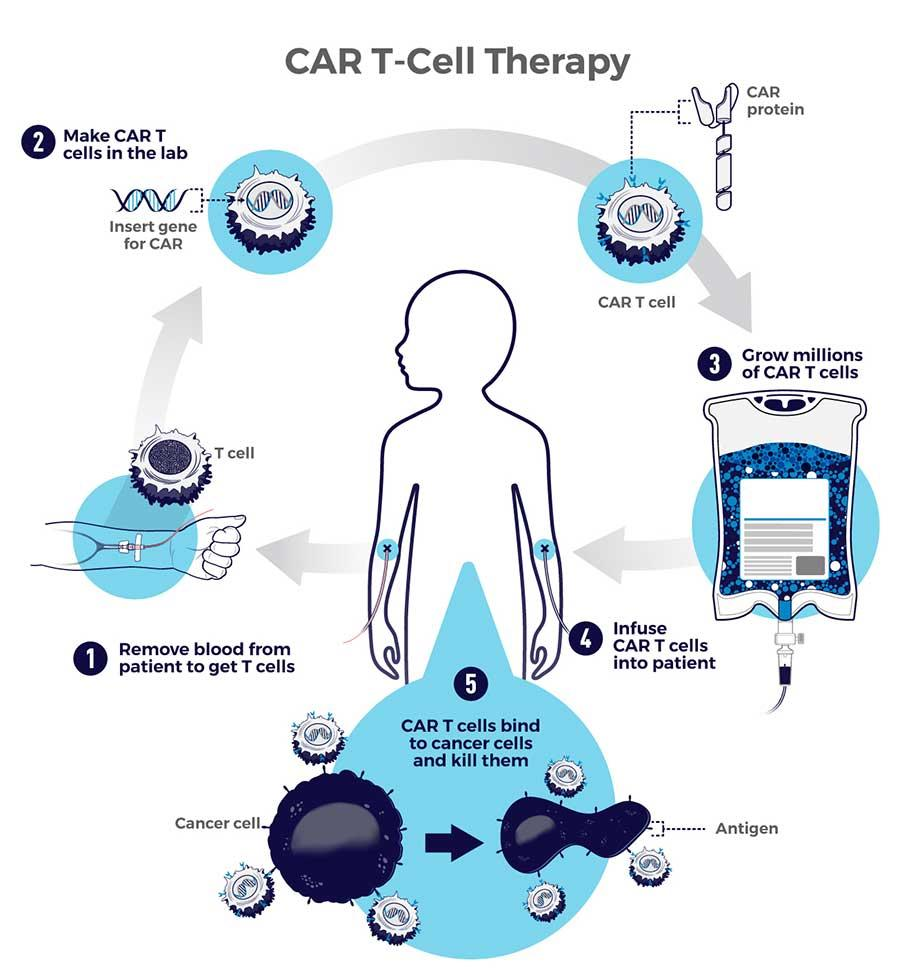
What is CAR T-Cell Therapy?
- About:
- CAR-T cell Therapy, also known as Chimeric Antigen Receptor T-cell Therapy, is a type of immunotherapy that uses a patient's own immune system to fight cancer.
- CAR T-cell therapy has been approved for leukaemias (cancers arising from the cells that produce white blood cells and lymphomas (arising from the lymphatic system).
- CAR-T cell therapies, often referred to as 'living drugs’.
- Since 2017, six CAR T-cell therapies have been approved by the Food and Drug Administration (FDA).
- All are approved for the treatment of blood cancers, including lymphomas, some forms of leukemia, and, most recently, multiple myeloma.
- Procedure:
- It is a complex and personalized treatment process that involves:
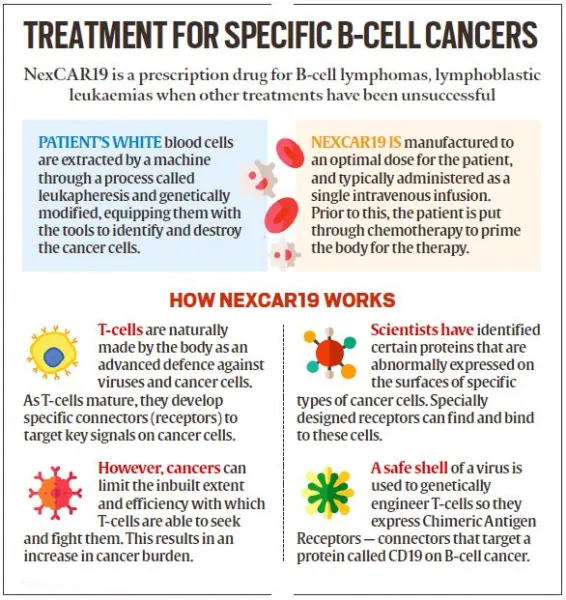
- Collecting T cells: T cells, a type of white blood cell that helps fight infection, are extracted from the patient's blood through a process known as Apheresis.
- Genetic Engineering: In the laboratory, the T cells are genetically modified to express a special protein called a Chimeric Antigen Receptor (CAR) on their surface.
- This CAR is designed to recognise and bind to a specific antigen (marker) found on cancer cells.
- Expansion: The engineered T cells are multiplied in large numbers in the lab.
- Infusion: The expanded CAR-T cells are then infused back into the patient's bloodstream, where they can identify and attack cancer cells that express the targeted antigen.
- It is a complex and personalized treatment process that involves:
- Development in India:
- NexCAR19, India's first indigenous CAR-T cell therapy for cancer, was developed collaboratively by ImmunoACT, Indian Institute of Technology Bombay (IIT-B), and Tata Memorial Hospital.
- The commercial use of this therapy to treat certain blood cancers was approved by the Central Drugs Standard Control Organisation (CDSCO) in October 2023.
- NexCAR19 is the first CAR-T cell therapy to get CDSCO approval.
- Potential Benefits of CAR-T Therapy:
- High Remission Rates: For some patients with advanced cancers who have not responded to other treatments, CAR-T therapy can lead to high rates of complete remission.
- Targeted Treatment: CAR T-cell therapy is highly targeted, as it specifically recognizes and attacks cancer cells expressing the target antigen while sparing healthy cells. This precision can lead to more effective treatment with fewer side effects compared to traditional chemotherapy and radiation therapy.
- High Efficacy: CAR T-cell therapy has shown remarkable efficacy, particularly in patients with certain types of blood cancers such as Acute Lymphoblastic Lukemia (ALL), Chronic Lymphocytic Leukemia (CLL), and Non-Hodgkin Lymphoma (NHL). It has achieved high rates of complete remission in some patients who have not responded to other treatments.
- Single Treatment: In many cases, CAR T-cell therapy involves a single infusion of genetically engineered T cells, which can provide long-lasting therapeutic effects. This contrasts with other treatments, such as chemotherapy, which may require multiple cycles of treatment over an extended period.
- Personalized Medicine: CAR T-cell therapy can be tailored to each individual patient by engineering T cells to target specific antigens present on their cancer cells. This personalized approach holds promise for treating a wide range of cancers and overcoming tumor heterogeneity.
What is Cancer?
- Cancer is a broad term used to describe a group of diseases characterized by the uncontrolled growth and spread of abnormal cells in the body.
- These abnormal cells, known as cancer cells, can invade nearby tissues and organs, disrupting their normal function.
- Additionally, cancer cells can metastasize, or spread to other parts of the body through the bloodstream or lymphatic system, forming new tumors in distant locations.
What is Gene Therapy?
- About:
- Gene therapy is a medical approach aimed at treating or preventing diseases by modifying the genetic material of a patient's cells.
- This technique involves introducing genetic material into a person's cells to either replace a faulty gene causing a disease or to provide a new function to the cells.
- Gene therapy holds promise for treating a wide range of genetic disorders, such as cystic fibrosis, muscular dystrophy, and certain types of cancer.
- Types of Gene Therapy:
- Gene Replacement Therapy: This involves inserting a healthy copy of a gene into the cells to replace a defective or missing gene.
- Gene Editing: Techniques like CRISPR-Cas9 enable precise editing of genes, allowing for corrections of mutations or modifications of gene expression.
- Gene Addition: In some cases, genes may be added to cells to help them function more effectively or to produce a beneficial protein.
- Gene Silencing: This approach involves inhibiting the expression of certain genes that may be causing disease by introducing molecules such as small interfering RNA (siRNA) or antisense oligonucleotides.
What is Vector?
- About: In gene therapy, a vector is typically a virus or a plasmid that has been modified to carry and deliver therapeutic genes into target cells.
- Viral Vectors: Viral vectors are derived from viruses that have been genetically engineered to remove their ability to cause disease while retaining their capacity to infect cells and deliver genetic material.
- Examples of viral vectors used in gene therapy include Lentiviruses, Adenoviruses, and Adeno-Associated Viruses (AAVs).
- Plasmid Vectors: Plasmid vectors are small, circular DNA molecules that can replicate independently within a host cell.
- They are commonly used in laboratory settings and experimental gene therapy approaches.
- Plasmid vectors can be introduced into target cells through methods such as electroporation or direct injection.
- Viral Vectors: Viral vectors are derived from viruses that have been genetically engineered to remove their ability to cause disease while retaining their capacity to infect cells and deliver genetic material.
What are the Challenges Regarding CAR T-Cell Therapy?
- Cytokine Release Syndrome (CRS): CRS is a systemic inflammatory response triggered by the activation and proliferation of CAR-T cells in the body.
- Symptoms can range from mild, flu-like symptoms to severe manifestations such as high fever, low blood pressure, and organ dysfunction. In severe cases, CRS can be life-threatening if not promptly managed.
- Cytopenias: Treatment with CAR T-cell therapy can lead to cytopenias, including low levels of red blood cells (anemia), white blood cells (neutropenia), and platelets (thrombocytopenia).
- These conditions can increase the risk of infections, bleeding, and other complications.
- Immune Effector Cell-Associated Syndrome (ICANS): ICANS encompasses a range of neurological symptoms associated with CAR T-cell therapy, including confusion, aphasia, and seizures. ICANS can occur concurrently with CRS or independently and may require close monitoring and intervention.
- Tumor Lysis Syndrome (TLS): In some cases, rapid destruction of cancer cells following CAR T-cell therapy can lead to the release of intracellular contents into the bloodstream, causing metabolic abnormalities such as hyperkalemia, hyperuricemia, and acute kidney injury.
Way Forward
- Cost Reduction:
- Explore strategies to reduce the high cost of CAR T-cell therapy, such as negotiating pricing agreements with manufacturers, implementing value-based pricing models, and investing in research and development to optimize manufacturing processes and increase efficiency.
- Management of Cytokine Release Syndrome (CRS):
- Develop standardized protocols for the early detection and management of CRS, including the use of immunosuppressive medications (such as tocilizumab) to dampen the inflammatory response.
- Enhance healthcare provider education and training on recognizing and managing CRS, including the importance of close monitoring and timely intervention.
- Management of Cytopenias:
- Implement strategies to mitigate the risk of cytopenias associated with CAR T-cell therapy, such as supportive care measures (e.g., blood transfusions, growth factors) and dose optimization to minimize hematologic toxicity while maintaining therapeutic efficacy.
- Management of Immune Effector Cell-Associated Syndrome (ICANS):
- Develop standardized approaches for the assessment and management of ICANS, including neurological monitoring and interventions (e.g., corticosteroids) for symptomatic relief.
- Invest in research to better understand the underlying mechanisms of ICANS and identify predictive biomarkers to guide risk stratification and early intervention.
- Prevention and Management of Tumor Lysis Syndrome (TLS):
- Implement protocols for the prevention and early detection of TLS, including hydration strategies and the use of urate-lowering agents.
- Monitor patients closely for signs of TLS during CAR T-cell therapy and provide prompt intervention to mitigate metabolic abnormalities and prevent renal complications.
UPSC Civil Services Examination, Previous Year Questions (PYQs)
Prelims
Q. Which one of the following statements best describes the role of B cells and T cells in the human body? (2022)
(a) They protect the environmental allergens. body
(b) They alleviate the body’s pain and inflammation.
(c) They act as immunosuppressants in the body.
(d) They protect the body from diseases caused by pathogens.
Ans: (d)
Mains
Q. What are the research and developmental achievements in applied biotechnology? How will these achievements help to uplift the poorer sections of the society? (2021)