Measures Related to Biosafety Regulations | 01 Jun 2020
Why in News
The Department of Biotechnology (DBT), Ministry of Science and Technology has taken measures to streamline the biosafety regulation for researchers and industries involved in Recombinant DNA Technology and management of hazardous microorganisms in the wake of Covid-19 pandemic.
Key Points
- Operationalization of Indian Biosafety Knowledge Portal:
- It was launched in May 2019 through which the Department receives all new applications related to research proposals. This has made the whole process transparent and time bound.
- It is a web based portal, with a major thrust to reach out to researchers, industry and other stakeholders to provide latest scientific information and regulatory guidance related to authorization of Genetically Modified Organisms/Living Modified Organisms (GMOs/LMOs) and products thereof.
- Institutional Biosafety Committee: This committee has been delegated authority to take decisions on applications of import, export and exchange of GMOs and product thereof for R&D purposes under the Environment (Protection) Act 1986.
- Facilitation of Research and Development on Covid-19: The DBT has proactively taken several steps to facilitate researchers and industries involved in research on Covid-19.
India's Biosafety and Recombinant DNA Guidelines
- Biosafety refers to policies and procedures adopted to avoid risk to human health and safety and to the conservation of the environment as a result of the use of GMOs for research and trade
- Under the Biosafety Research programme, main emphasis is given to facilitate the implementation of biosafety procedures, rules and guidelines under Environment (Protection) Act 1986 and Rules 1989 to ensure safety from the use of GMOs and products thereof in research and application to the users as well as to the environment.
- A three tier mechanism comprising Institutional Biosafety Committees (IBSC) at the Institute/company; the Review Committee on Genetic Manipulation (RCGM) in the Department of Biotechnology; and the Genetic Engineering Approval Committee (GEAC) in the Ministry of Environment & Forests (MoE&F) for granting approval for research and development activities on recombinant DNA products, environmental release of Genetically Engineered (GE) crops and monitoring and evaluation of research activities involving recombinant DNA technology has been established.
Recombinant DNA Technology
- The technology used for producing artificial Deoxyribonucleic Acid (DNA) through the combination of different genetic materials (DNA) from different sources is referred to as Recombinant DNA Technology.
- Recombinant DNA technology is popularly known as genetic engineering.
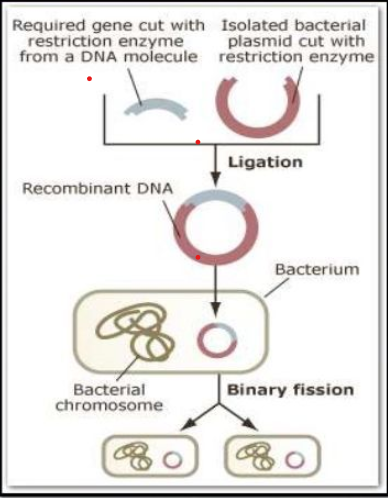
- Process of Recombinant DNA Technology: Recombinant DNA technology involves several steps in specific sequence:
- Isolation of Genetic Material: It involves the isolation of desired DNA in its purest form i.e. free from macromolecules.
- Cutting the gene at the recognition sites: The restriction enzymes determine the location at which the desired gene is inserted into the vector genome (a virus or plasmid that is used to ferry a desired DNA sequence into a host cell).
- Ligation of DNA Molecules: Ligation involves joining of the two pieces – a cut fragment of DNA and the vector together with the help of the enzyme DNA ligase.The DNA molecule thus produced is called recombinant DNA.
- Insertion of Recombinant DNA into Host: The recombinant DNA is introduced into a recipient host cell. This process is termed as Transformation.
- After the insertion of the recombinant DNA into the host cell, it gets multiplied and is expressed in the form of the manufactured protein under optimal conditions.
- The effectively transformed cells/organisms carry forward the recombinant gene to the offspring.
- The recombinant DNA molecule produces new genetic combinations that are of value to science, medicine, agriculture, and industry.
Application
- Gene Therapy: It is used as an attempt to correct the gene defects which give rise to heredity diseases.
- Clinical diagnosis–
- It has been useful in detecting the presence of Human immunodeficiency Virus in a person.
- Enzyme-Linked Immunosorbent Assay (ELISA) is an example of the application of recombinant DNA technology.
- Enzyme-Linked Immunosorbent Assay (ELISA) is a test that detects and measures antibodies in blood.
- Medicines: For the production of Insulin.
- Agriculture: To produce genetically-modified organisms such as Flavr Savr tomatoes.