Li-ion Battery Recycling Technology to Boost Circular Economy | 07 Jun 2023
Why in News?
The Ministry of Electronics and Information Technology (MeitY) in India has taken a significant step towards promoting a circular economy by transferring a cost-effective Li-ion battery recycling technology to nine recycling industries and start-ups.
- The technology was developed under the "Centre of Excellence on E-waste management" at the Centre for Materials for Electronics Technology (C-MET), Hyderabad, in collaboration with the Government of Telangana and industry partner M/s Greenko Energies Pvt. Ltd., Hyderabad.
- This initiative is part of the Mission Lifestyle for the Environment (LiFE) under the "Promote circularity campaign."
What is the Recently Invented Recycling Technology?
- The recycling technology for Li-ion batteries is designed to efficiently process and recover valuable materials from discarded batteries.
- The process begins by soaking the batteries in a solution to extract the valuable metals.
- The solution aids in the separation and extraction of metals such as Lithium (Li), Cobalt (Co), Manganese (Mn), and Nickel (Ni), enabling the recovery of over 95% of their contents in the form of corresponding oxides/carbonates with a purity of approximately 98%.
- These metals are then transformed into their pure forms, ready to be reused in making new batteries or other useful applications.
- This technology ensures that over 95% of these valuable metals are recovered from batteries.
- By recycling the batteries, we can reduce the need for mining new resources and contribute to a more sustainable environment.
- The recycling technology for Li-ion batteries plays a crucial role in promoting a circular economy.
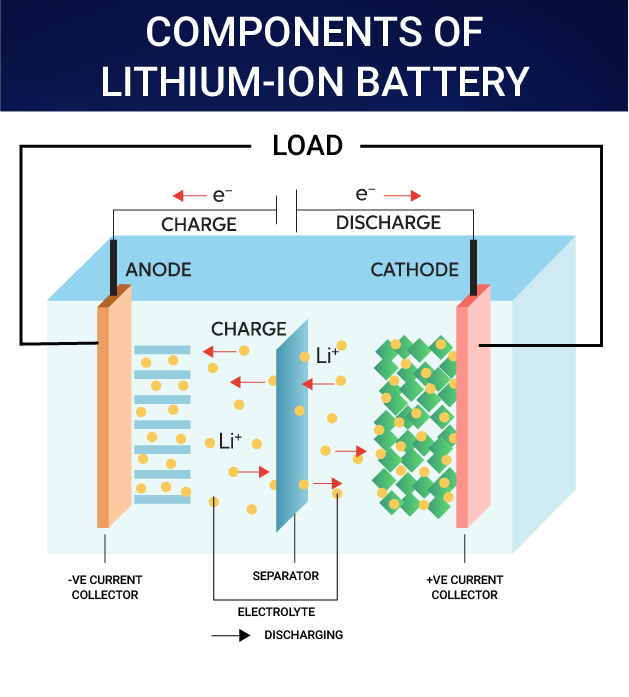
What is a Li-ion Battery?
- About:
- A lithium-ion (Li-ion) battery is a type of rechargeable battery.
- Li-ion batteries use an intercalated (Intercalation is the reversible inclusion or insertion of a molecule into materials with layered structures) lithium compound as one electrode material, compared to the metallic lithium used in a non-rechargeable lithium battery.
- The battery consists of an electrolyte, which allows for ionic movement, and the two electrodes are the constituent components of a lithium-ion battery cell.
- Lithium ions move from the negative electrode to the positive electrode during discharge and back when charging.
- Applications:
- Electronic gadgets, Tele-communication, Aerospace, Industrial applications.
- Lithium-ion battery technology has made it the favourite power source for electric and hybrid electric vehicles.
- Disadvantages of Li-ion Batteries:
- Long charging times.
- Safety issues as instances of batteries catching fires have been there.
- Expensive to manufacture.
- While the Li-ion batteries are seen as sufficiently efficient for applications such as phones and laptops, in case of EVs, these cells still lack the range that would make them a viable alternative to internal combustion engines.
What is Lithium?
- About:
- Lithium (Li), sometimes also referred as ‘White gold’ due to its high demand for rechargeable batteries, is a soft and silvery-white metal.
- Extraction:
- Lithium can be extracted in different ways, depending on the type of the deposit — generally either through solar evaporation of large brine pools, or from hard-rock extraction of the ore.
- Uses:
- Lithium is an important component of electrochemical cells used in batteries of EVs, Laptops, Mobiles etc.
- It is also used in thermonuclear reactions.
- It is used to make alloys with aluminium and magnesium, improving their strength and making them lighter.
- Magnesium-lithium alloy - for armour plating.
- Aluminum-lithium alloys - in aircraft, bicycle frames and high-speed trains.
- Major Global Lithium Reserves:
- Chile > Australia > Argentina are top countries with Li reserves.
- Lithium Triangle: Chile, Argentina, Bolivia.
- Lithium Reserves in India:
- Preliminary survey showed estimated lithium reserves of 14,100 tonnes in a small patch of land surveyed in Southern Karnataka’s Mandya district.
- Other potential sites:
- Mica belts in Rajasthan, Bihar, Andhra Pradesh.
- Pegmatite belts in Odisha and Chhattisgarh.
- Rann of Kutch in Gujrat.
UPSC Civil Services Examination, Previous Year Question (PYQ)
Q. Which one of the following pairs of metals constitutes the lightest metal and the heaviest metal, respectively? (2008)
(a) Lithium and mercury
(b) Lithium and osmium
(c) Aluminium and osmium
(d) Aluminium and mercury
Ans: (b)
Exp:
- Light metals are metals of low atomic weight while heavier elements generally have high atomic weight.
- Osmium is a hard metallic element which has the greatest density of all known elements. Osmium has an atomic weight of 190.2 u and its atomic number is 76.
- Lithium having an atomic number 3 and atomic weight of 6.941u is the lightest known metal.
- Therefore, option (b) is the correct answer.