DCGI Directives on Global Standard GMP | 13 Nov 2025
For Prelims: Drugs Controller General of India, World Health Organization, Central Drugs Standard Control Organisation, National Pharmaceutical Pricing Authority
For Mains: Drug regulation reforms under the Drugs and Cosmetics Act, Public health and pharmaceutical safety, India’s role as Pharmacy of the World
Why in News?
The Drugs Controller General of India (DCGI) has instructed state drug regulators to enforce revised Good Manufacturing Practices (GMP) under Schedule M of the Drugs and cosmetic act 1940 and rules 1945, by January 2026, aligning India’s pharmaceutical manufacturing norms with global standards.
- The move comes amid heightened scrutiny following incidents of contaminated cough syrups that caused several child deaths in India and abroad.
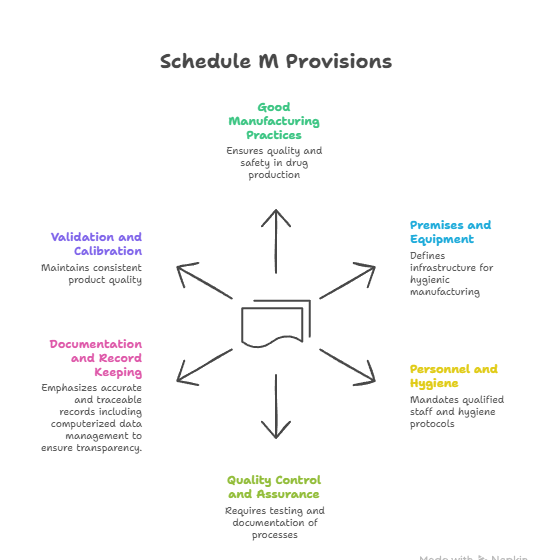
What is the Schedule M of the Drugs and Cosmetic Act 1940?
- About: The Schedule M under the Drugs and Cosmetics Act, 1940 (and the corresponding Drugs and Cosmetics Rules, 1945) serves as the statutory framework that ensures all medicines produced in the country are safe, effective, and of consistent quality, both for domestic use and export.
- In 2023, the Centre revised Schedule M requirements wherein “GMP” was upgraded to “good manufacturing practices and requirements of plan and equipment for pharmaceutical products” to align India’s drug manufacturing standards with World Health Organization (WHO) -GMP norms.
- Key Provisions of Schedule M:
What is the Current State of India's Pharmaceutical Sector?
- India as Pharmacy of the World: India is the world’s 3rd largest producer of medicines by volume and 14th by value.
- It is also the largest supplier of generic drugs, accounting for about 20% of global exports, and a major source of affordable vaccines worldwide, reinforcing its status as the “Pharmacy of the World.”
- India’s pharma sector supplies 55-60% of UNICEF’s vaccines, meeting 99% of WHO’s DPT (Diphtheria, Whooping cough and Tetanus) vaccine demand, 52% for BCG (Bacillus Calmette-Guérin is a vaccine primarily used against tuberculosis (TB)), and 45% for measles.
- From Africa to America, Indian vaccines save millions.
- India’s Biotechnology Sector: India’s biotech industry expanded from USD 10 billion (2014) to over USD 130 billion in 2024 ( a 13-fold increase). It is projected to reach USD 300 billion by 2030.
- Economic Potential: Drug and pharma exports rose from USD 15.07 billion (2013–14) to USD 27.85 billion in 2023–24. The US, Belgium, South Africa, the UK, and Brazil are the top five export destinations.
- The Indian pharmaceutical industry is projected to grow at a CAGR of over 10% to reach a size of USD 130 billion by 2030.
- India has become a global hub for medical tourism, offering affordable, high-quality treatment enabled by major reforms in the healthcare sector.
What are the Challenges Faced by India’s Pharmaceutical Industry?
- Quality Control and Compliance Issues; India has witnessed several incidents of substandard and contaminated drugs, including cough syrup tragedies in India and abroad.
- Many small and medium manufacturers struggle to fully comply with GMP.
- Several countries have flagged contamination and safety issues, and repeated quality lapses along with WHO warnings have hurt India’s credibility as a dependable drug producer.
- Compliance with global certifications such as the US Food and Drug Administration's (FDA) has become more expensive, putting financial strain on small exporters.
- Regulatory Weaknesses: The Indian regulatory system remains fragmented, divided between the Central Drugs Standard Control Organisation (CDSCO) and state-level drug authorities.
- This overlap leads to inconsistent enforcement and delays in approvals.
- Despite the introduction of revised Schedule M (2023) to align with global norms, the pace of implementation has been slow, creating uneven standards across regions.
- Dependence on Imported Raw Materials: India imports nearly 70% of its Active Pharmaceutical Ingredients (APIs), mainly from China, which poses major risks to supply chain security and price stability.
- The Covid-19 pandemic exposed this overdependence, disrupting domestic production.
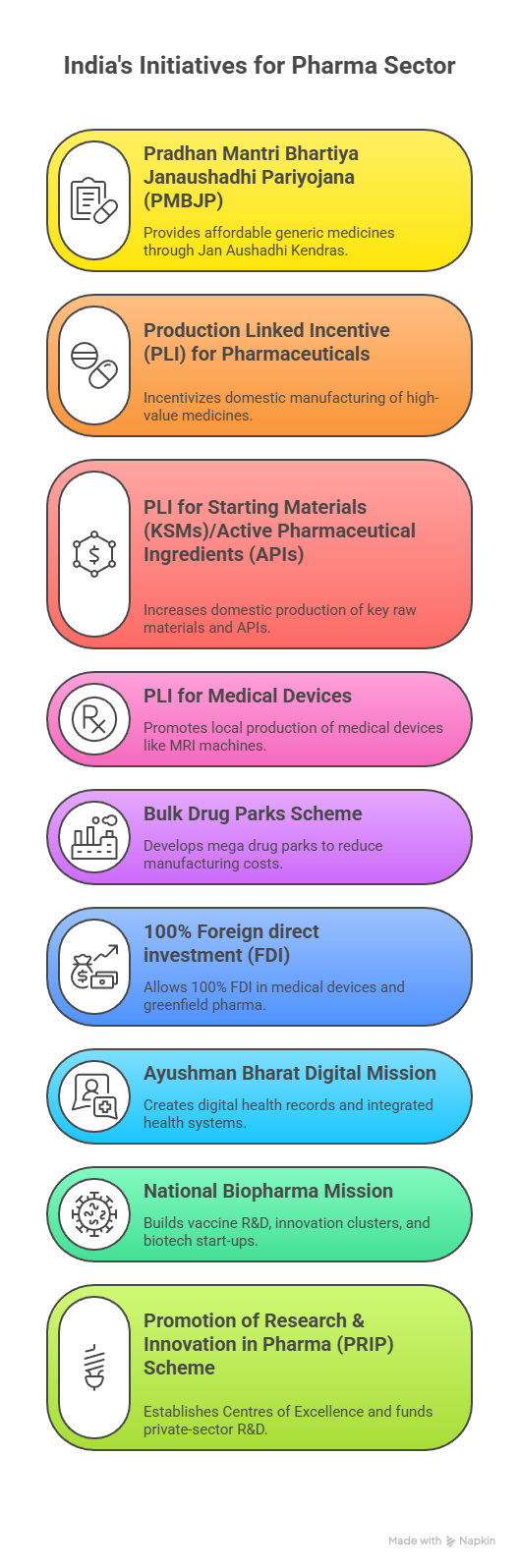
- Although the government launched the PLI Scheme for Bulk Drugs to promote local manufacturing, progress remains limited.
- Price Controls and Low Profit Margins: Strict price caps imposed by the National Pharmaceutical Pricing Authority (NPPA) keep essential medicines affordable but reduce profit margins for companies.
- This leaves little room for investment in R&D or technological upgrades. As a result, firms often prioritise cost efficiency over innovation and quality improvements.
- Weak R&D: Most Indian pharmaceutical companies focus on generic production rather than developing new drugs or molecules.
- Limited collaboration between academia, industry, and government further restricts innovation and slows the creation of high-value, patented drugs.
- Emerging and Technological Challenges: The industry also faces new issues such as antimicrobial resistance (AMR), which is linked to excessive antibiotic use.
- The rise of biopharmaceuticals, AI-driven drug discovery, and green manufacturing requires rapid technological adaptation.
- Companies must upgrade facilities and expertise to remain globally competitive and environmentally sustainable.
What Measures can Strengthen India’s Pharmaceutical Sector?
- Strengthen GMP Compliance: Upgrade infrastructure of MSME pharma units through subsidised loans and technology grants.
- Mandatory third-party audits and surprise inspections to ensure strict Schedule M and WHO-GMP compliance.
- Modernise Drug Regulation: Establish a single, centralised national drug authority to streamline approvals and avoid CDSCO–state overlaps.
- Introduce uniform digital platforms for licensing, batch tracking, and adverse drug reaction reporting.
- Reduce Dependency on Imported APIs: Fast-track the Bulk Drug Parks and PLI schemes to revive domestic API and intermediate manufacturing. Provide long-term purchase guarantees or viability-gap funding for critical APIs.
- Boost R&D, Innovation & Drug Discovery: Increase public investment in pharma R&D to at least 2% of GDP, with grants for start-ups and academia.
- Strengthen National Institutes of Pharmaceutical Education and Research, biotech parks, and Centres of Excellence under the PRIP Scheme.
- Improve Export Competitiveness: Support small exporters in meeting USFDA, and EU-GMP standards through training and financial subsidies.
- Negotiate regulatory harmonisation agreements with Africa, Latin America, and ASEAN markets.
- Strengthen Waste Management Standards: India should tighten pharma effluent norms to curb AMR, promote green manufacturing and solvent recovery, and develop zero-liquid-discharge clusters for cleaner, sustainable production.
Conclusion
India’s pharmaceutical industry stands at a crossroads, admired globally for affordable generics but under scrutiny for quality, safety, and innovation gaps. To maintain its status as the “Pharmacy of the World,” India must strengthen regulatory enforcement, reduce API dependence, invest in research and digital innovation, and support small manufacturers in meeting international standards.
|
Drishti Mains Question: “Revised Schedule M marks a major step towards global-standard manufacturing, but India’s pharma sector needs deeper structural reform.” Discuss. |
Frequently Asked Questions (FAQs)
1. What is Schedule M under the Drugs and Cosmetics Act?
It sets Good Manufacturing Practices (GMP) and infrastructure standards for pharmaceutical units under the Drugs and Cosmetics Act; revised in 2023 to align with WHO-GMP norms.
2. What is India’s global status in pharmaceuticals?
India is the 3rd-largest producer by volume, supplies ~20% of global generics, and provides 55–60% of UNICEF vaccines.
3. Who is the Drugs Controller General of India (DCGI)?
The DCGI heads the Central Drugs Standard Control Organisation (CDSCO), which is responsible for ensuring quality drugs supply across the country.
UPSC Civil Services Examination, Previous Year Question (PYQ)
Prelims
Q. Which of the following are the reasons for the occurrence of multi-drug resistance in microbial pathogens in India? (2019)
- Genetic predisposition of some people
- Taking incorrect doses of antibiotics to cure diseases
- Using antibiotics in livestock farming
- Multiple chronic diseases in some people
Select the correct answer using the code given below.
(a) 1 and 2
(b) 2 and 3 only
(c) 1, 3 and 4
(d) 2, 3 and 4
Ans: (b)
Mains
Q. How is the Government of India protecting traditional knowledge of medicine from patenting by pharmaceutical companies? (2019)